Coelenterates are multicellular animals with ray (radial) symmetry. Their body consists of two layers of cells and has a sac-like, so-called intestinal cavity. Coelenterates are characterized by the presence of special stinging cells.
Radiation symmetry is a characteristic general feature of sessile or sedentary animals. In this case, the animal can be equally in danger from any side, and food also comes from all sides. Therefore, the bodies of these animals are designed in such a way that the means of protection or catching prey are directed in different directions, like rays (or radii) from a single center.
Coelenterates are the most ancient and primitive multicellular animals. They evolved from primitive primordial multicellular organisms.
All coelenterates are aquatic animals, most of which live in the seas and oceans. They inhabit seas from the surface to extreme depths, from tropical waters to polar regions. A small number of species live in fresh waters. About 9,000 species of coelenterates are now known. Among them there are solitary and colonial animals.
A group of individuals that have similar adaptations for living in the same environment is called a life form of animals. Modern coelenterates are characterized by two life forms (two generations): an attached form - a polyp and a free-swimming form - a jellyfish.
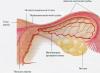
Polyps (from the Greek polyp - “multipede”) - a life form, so named for its numerous tentacles. In rare cases (Fig. 36, A) polyps are single (for example, hydra and sea anemone), but more often they form colonies of up to several thousand individuals. In the form of a jellyfish (Fig. 36, B), coelenterates, as a rule, live solitarily.
Rice. 36. Schemes of the structure of coelenterates: A - polyp; B - jellyfish
In many coelenterates, both life forms (both generations) replace each other (alternate) during the life cycle - from the birth of the organism to death. Some (hydra, coral polyps) do not have a free-swimming form - jellyfish. Others (some scyphoid jellyfish; see next paragraph) have lost their polyp form.
The body of coelenterates resembles a two-layer sac open at one end. The outer layer of cells is called ectoderm (from the Greek ectos - “outside” and dermis - “skin”), and the inner layer is called endoderm (from the Greek entos - “inside” and dermis - “skin”). The only body cavity of these animals, the intestinal cavity, communicates with the external environment through the oral opening (mouth). Through the mouth, food enters the intestinal cavity, and undigested residues are expelled through it.
In coelenterates, stinging cells are located on the tentacles. They serve both for catching prey and for defense. Coelenterates are predators. They feed on various small animals that “float” in the water column.
Coelenterates reproduce both asexually and sexually.
The importance of coelenterates
Coelenterates are of great importance in nature. Many fish feed on coral polyps and hide among the limestone, branched “forests” built by these animals. Sea turtles and some fish feed on jellyfish. The coelenterates themselves, being predators, influence marine animal communities by eating planktonic organisms, and large sea anemones and jellyfish also eat small fish. Humans use some coelenterates. Dead calcareous parts of coral reefs in some coastal countries are used for construction material and lime is produced by burning. Some types of jellyfish are edible. Black and red corals are used to make jewelry.
Some swimming jellyfish, sea anemones and corals with stinging cells can cause severe burns to fishermen, divers and swimmers. Coral reefs impede shipping in some areas.
Freshwater hydra - single polyp
Habitat. External building. Lifestyle. Movement. Freshwater hydra lives in areas with clean water (in river backwaters, lakes and ponds) (Fig. 37). This is a small translucent animal about 1 cm long. The body of the hydra has a cylindrical shape. With its lower end (sole) it is attached to the stems and leaves of aquatic plants, snags and stones. At the upper end of the body, around the mouth, there are 6-12 tentacles. Hydra, like other coelenterates, is characterized by radial symmetry. Hydra leads a sedentary lifestyle. Its body and tentacles can lengthen and shorten. In a calm state, the tentacles extend several centimeters. The animal slowly moves them from side to side, lying in wait for prey. If necessary, the hydra can move slowly. At the same time, it seems to be walking (tumbling), attaching itself to underwater objects alternately with the upper and lower ends of the body.

Rice. 37. Scheme of the structure of freshwater hydra: 1 - ectoderm; 2 - endoderm; 3 - mesoglea: 4 - sole; 5 - intestinal cavity; 6 - kidney; 7 - tentacle; 8 - mouth
The hydra's body looks like an oblong sac, the walls of which consist of two layers of cells - ectoderm and endoderm. Between them lies a thin gelatinous non-cellular layer - mesoglea, which serves as a support. The intestinal cavity of the hydra communicates with the external environment only through the mouth.
The ectoderm forms the covering of the animal’s body and consists of several types of cells (Fig. 38). The most numerous of them are epithelial-muscular. Due to the muscle fibers lying at the base of each cell, the hydra's body can contract, lengthen and bend.

Rice. 38. Section through the body of the hydra - ectoderm cells (1-4) and endoderm (5, 6): 1 - epithelial-muscle cells; 2 - intermediate cell; 3 - stinging cell; 4 - nerve cell; 5 - digestive-muscle cell; 6 - glandular cell; 7 - mesoglea
The ectoderm contains star-shaped nerve cells. The processes of neighboring nerve cells connect with each other, forming a nerve network that covers the entire body of the animal and represents the most primitive nervous system in animals.
If you touch the hydra or prick it with a needle, the animal will shrink. This happens because the signal received by even one cell will spread throughout the entire nerve network. Nerve cells “give command” to epithelial muscle cells. The muscle fibers contract, and then the entire body of the hydra shortens (Fig. 39). The response of the hydra body to such irritation is an example of an unconditioned reflex. Unconditioned reflexes are characteristic of all multicellular animals.

Rice. 39. Stimulation of hydra nerve cells
The ectoderm also contains stinging cells that serve for attack and defense. They are mainly located on the tentacles of the hydra. Each stinging cell contains an oval capsule in which the stinging filament is coiled. If prey or an enemy touches the sensitive hair, which is located outside the stinging cell, in response to irritation the stinging thread is ejected and pierces the body of the victim (Fig. 40). Through the thread channel, a substance that can paralyze it enters the victim’s body.

Rice. 40. Diagram of the structure of a stinging cell: 1 - nucleus; 2 - stinging capsule; 3 - sensitive hair; 4 - stinging thread: coiled (left) and thrown out (right)
There are several types of stinging cells. The threads of some pierce the skin of animals and introduce poison into their bodies. The threads of others are wrapped around the prey. The threads of the third are very sticky and stick to the victim. Usually the hydra “shoots” several stinging cells.
The ectoderm also contains intermediate cells. From them other types of cells are formed.
Endoderm lines the entire intestinal cavity from the inside. The endoderm consists of digestive muscle and glandular cells. There are more digestive muscle cells than others. Their muscle fibers are capable of contraction. When they shorten, the hydra's body becomes thinner. Complex movements, for example the hydra's "tumbling" movement, occur due to contractions of the muscle fibers of the ectoderm and endoderm cells.
Nutrition. Each of the digestive-muscle cells of the endoderm has one to three flagella. Vibrating flagella create a current of water, which drives food particles towards the cells. The glandular cells present in the endoderm secrete digestive juice into the intestinal cavity, which liquefies and partially digests food.
Digestive muscle cells of the endoderm are capable of forming pseudopods, capturing and digesting small food particles in the digestive vacuoles. Thus, digestion in hydra and all coelenterates is intracellular and cavity.
Nutrients are distributed throughout the hydra's body. Undigested residues are removed through the mouth. Hydras feed on small invertebrate animals (often crustaceans - daphnia and cyclops), which they catch with their tentacles.
Breathing and excretion. Hydra breathes oxygen dissolved in water. She has no respiratory organs, and she absorbs oxygen over the entire surface of her body, releasing carbon dioxide. During the process of life, harmful substances are formed in cells and released into the water.
Reproduction and development. Hydra reproduces sexually and asexually. Asexual reproduction is carried out by budding (Fig. 41). A protrusion forms on the body of the hydra - a kidney. It consists of two layers of cells - ectoderm and endoderm - and communicates through a common cavity with the maternal body. The kidney enlarges and grows in length. A mouth and small tentacles appear at its top, and a sole appears at the base. After this, the young hydra separates from the mother’s body, sinks to the bottom and begins an independent lifestyle. Often several buds form on the hydra’s body at once. Hydras most often reproduce asexually.

Rice. 41. Asexual reproduction of hydra (budding)
In autumn, with the approach of cold weather, hydras begin to reproduce sexually (Fig. 42). Sex cells are formed in the ectoderm from intermediate cells. Two types of tubercles appear on the body of the hydra. In some, sperm are formed, in others, eggs.

Rice. 42. Hydra sexual reproduction
There are hydras in which sperm and eggs are formed on different individuals. These are dioecious animals. In other species of hydras, both sperm and eggs are formed on the body of one organism. Such animals, combining the characteristics of both female and male genders, are called hermaphrodites.
Once in the water, the sperm swims with the help of a long flagellum and reaches the immobile eggs. Fertilization (the union of a sperm with an egg) occurs in the body of the mother's body. After this, a dense membrane is formed around the fertilized egg. A fertilized egg divides multiple times to form an embryo. In the fall, the hydra dies, and the shell-covered embryos sink to the bottom. In spring, embryo development continues. After the reservoir warms up, the membranes covering the embryos are destroyed and small hydras come out.
Regeneration. A damaged hydra easily restores lost body parts (Fig. 43) not only after it has been cut in half, but even if it has been dismembered into many parts. From each part a new small hydra is formed. This occurs due to the intensive division of intermediate cells, from which other types of cells arise. The ability of animals to restore damaged or lost body parts is called regeneration.

Rice. 43. Hydra regeneration
Hydra is a multicellular animal with a primitive structure. Her intestinal cavity looks like a solid bag. The nervous system consists of scattered stellate nerve cells that form the nerve network. Asexual reproduction occurs by budding. Hydra also reproduces sexually.
Exercises based on the material covered
- List the main structural features of representatives of the phylum Coelenterata.
- What is the significance of the different cell types in Hydra?
- Describe radial symmetry using the example of one of the representatives of the coelenterate phylum.
- What kind of life do coelenterates lead?
- How does a hydra move?
- Describe the vital functions of coelenterates: nutrition, digestion, reproduction (using the example of hydra).
- Explain, using a picture, the regeneration process of the hydra.
- What is the significance of coelenterates?
Coelenterates are considered the first true multicellular animals. In the process of their individual development, two germ layers are formed - endoderm and ectoderm. For comparison, sponge larvae do not form such germ layers. In the body of coelenterates, tissues and even organs can be distinguished.
Coelenterates were considered earlier and are now considered in many textbooks in the rank type. However, in modern literature, coelenterates are increasingly considered a group without a systematic rank, and under this name they combine two types whose representatives live in our time - the Cnidarians type and the Ctenophores type. The old view of coelenterates did not include ctenophores. In this case, Coelenterates and Cnidarians are one and the same. Cnidarians include Hydroids, Scyphoids, Coral polyps and some other classes. The description given below primarily applies to cnidarians.
All coelenterate aquatic animals, most of which live in the seas and oceans. A small part lives in freshwater bodies.
Coelenterates - radially symmetrical animals. This distinguishes them from more complexly organized animals that have bilateral symmetry. In the case of radial (or radial) symmetry, many planes can be drawn through the body, which will divide it into two symmetrical halves. In the case of bilateral symmetry, there can be only one such plane.
The radial symmetry of the animal suggests that it does not care from which side the food swims towards it. This symmetry is beneficial for a sedentary lifestyle. This is the way of life of many coelenterates. However, among them there are also mobile radially symmetrical forms (jellyfish).
Coelenterates (cnidarians) are characterized by two life forms - polyp and jellyfish. Polyp leads an attached lifestyle, often forms colonies, but there are also solitary forms. Jellyfish- a mobile life form, in its general structure it resembles a somewhat flattened polyp, which is inverted with its mouth opening and tentacles down. In the life cycle of many coelenterates, an alternation of life forms occurs: polyps form jellyfish, which reproduce sexually, after which young polyps are formed, which, upon becoming adults, reproduce by budding (asexual method), and also form jellyfish. However, a number of coelenterate species are represented by only one life form.
Coelenterates have tentacles surrounding the mouth, which serve them to catch, capture and place food in the mouth. All animals of this type are predators (food includes arthropods, unicellular organisms, small fish, etc.). The tentacles can also serve as organs of locomotion.
In the process of evolution, a number of progressive features appeared in the structure of coelenterates in comparison with colonial protozoa and sponges. This is how they developed an intestinal cavity. Food is partially digested in it, and not exclusively in cells, like in sponges. If in sponges the channels and cavities simply pumped water, from which the cells captured food particles, then in coelenterates the cavity was essentially turned into a digestive system.
The intestinal cavity has only one oral opening. Removal of undigested food residues occurs through it.
Coelenterates have two layers of cells - ectoderm(external) and enToderma(interior). They can also be considered types of fabrics. Although there are more types of cells. Between the ectoderm and endoderm there is an intercellular layer mesoglea, consisting of a gelatinous substance. In jellyfish, the mesoglea is more developed.
The ectoderm contains most skin-muscle cells. Eat stinging cells, which “shoot” when defending or attacking. Coelenterates do not have muscle tissue, but ectoderm and endoderm cells have muscular appendages, which are located between these layers. Their contraction and relaxation, controlled by the nervous system, determines the coordinated movement of parts of the animal’s body and its movement in space.
The endoderm mainly consists of cells responsible for digesting food, as well as secretory, releasing substances into the intestinal cavity for extracellular digestion.
The unity of the body's reactions to environmental stimuli is carried out thanks to the nervous system. In coelenterates, an unconditioned reflex already appears. The nervous system is diffuse (mesh). Its cells are located throughout the body at the base of the ectoderm. But in jellyfish you can observe nerve ganglia, light-sensitive organs and balance organs.
Coelenterates can reach quite large sizes. But still, they do not have respiratory or excretory organs. The double layer provides contact with the external environment for almost all cells, allowing them to absorb and release directly into the water around them.
In the body of coelenterates there is Pintermediate cells, capable of dividing and turning into all other types of cells. This is due to the greater ability of these animals to regenerate (restore lost body parts).
Biological processes in living organisms
1.
Biology and systematics. Roundworms
Human biological rhythms
2.1 Test to determine the type of performance
Equipment: self-assessment table (Appendix 1).
By answering the test questions, choosing the appropriate answers to the questions asked and calculating the points, you can determine the type of performance...
4. Generality of 1/f type processes (flicker noise type processes) for all systems
The general process for any systems existing in the frequency range from 1024 (proton) to 10-17 (Universe) Hz is a 1/f type process, for which the parameter value of the observed event is inversely proportional to the frequency of the event...
Dependence of the spatiotemporal structure of an open system and its statistical properties on time
5.
The role of 1/f type processes in economics
The role of 1/f type processes in business, econometrics and finance is very large, and this explains the large number of publications. The 1/f type relationship describes the economic systems and processes listed below in the following articles...
Dependence of the spatiotemporal structure of an open system and its statistical properties on time
New mathematical description of 1/f type processes. Spectroscopy of flicker noise processes (SFN - spectroscopy of 1/f type processes)
Dynamic structure of a nonlinear system, including the market...
Features of earthworms
1. Type annelids (Annelida)
The phylum annelids, or annelids, covers a significant number of species (about 9000) of higher worms.
The main characteristics of the type Annelida are as follows: 1. The body of annelids is composed of the head lobe (prostomium)…
Type annelids
1. Class Polychaete worms
The body of polychaete ringlets has various appendages: parapodia, sensitive antennae, setae - they serve for movement and are sensory organs.
The appendages on the head section are more developed...
Type annelids
2. Class Oligochaete worms
The class of oligochaetes includes annelids, which have the basic features of the type, but with underdeveloped tentacles, parapodia and gills.
This is due to adaptation to life in the sandy soils of reservoirs (tubifex) and in the soil (earthworms)…
Type flatworms
Class Ciliated worms
Most eyelash worms are free-living animals that, as a rule, lead a predatory lifestyle. They eat many protozoa (ciliates, rhizomes, flagellates), nematodes, small crustaceans...
Type flatworms
Class Tapeworms
The class includes about 3 thousand.
Two-layer animals
Flatworms are three-layered animals; they develop mesoderm located between the ectoderm and endoderm.
2.2 Class Tapeworms
2.3 Flatworms (Plathelminthes)
The type of flatworms includes the most poorly organized three-layered bilateral animals leading a diverse lifestyle.
Flatworms have a number of features in their structure and biology, which developed under the influence of living conditions...
Evolution of the excretory system of invertebrates
2.5 Roundworms (Nemathelminthes)
This type is characterized by either the absence of an excretory system, or it is represented by modified skin glands, or it is of the protonephridial type...
Evolution of the excretory system of invertebrates
2.6 Annelida
The phylum annelids, or annelids, are a very important group for understanding the evolution of higher invertebrates.
The main feature of the external structure of the rings is metamerism, or body segmentation...
|
The animal world is large and diverse. Animals are animals, but adults decided to divide them all into groups according to certain characteristics. The science of classifying animals is called systematics or taxonomy.
This science determines family relationships between organisms. The degree of relationship is not always determined by external similarity. For example, marsupial mice are very similar to ordinary mice, and tupayas are very similar to squirrels. However, these animals belong to different orders. But armadillos, anteaters and sloths, completely different from each other, are united into one squad. The fact is that family ties between animals are determined by their origin.

Types of multicellular animals: sponges, bryozoans, flatworms, roundworms and annelids (worms), coelenterates, arthropods, molluscs, echinoderms and chordates.
Chordates are the most progressive type of animals. They are united by the presence of a chord - the primary skeletal axis.
Characteristics of some types of animals
The most highly developed chordates are grouped into the vertebrate subphylum. Their notochord is transformed into a spine. The rest are called invertebrates.

Types are divided into classes. There are 5 classes of vertebrates in total: fish, amphibians, birds, reptiles (reptiles) and mammals (animals).
Mammals are the most highly organized animals of all vertebrates.
Classes can be divided into subclasses. For example, mammals are divided into subclasses: viviparous and oviparous.

The classifications are approximate and change all the time. For example, now lagomorphs have been moved from rodents into an independent order.
In fact, those groups of animals that are studied in elementary school are types and classes of animals, given intermixed.

The first mammals appeared on Earth about 200 million years ago, separating from animal-like reptiles.







The science of classifying animals is called systematics or taxonomy. This science determines family relationships between organisms. The degree of relationship is not always determined by external similarity. For example, marsupial mice are very similar to ordinary mice, and tupai are very similar to squirrels. However, these animals belong to different orders. But armadillos, anteaters and sloths, completely different from each other, are united into one squad. The fact is that family ties between animals are determined by their origin.
By studying the skeletal structure and dental system of animals, scientists determine which animals are closest to each other, and paleontological finds of ancient extinct species of animals help to more accurately establish family ties between their descendants.
Plays a major role in the taxonomy of animals genetics- the science of the laws of heredity.
The first mammals appeared on Earth about 200 million years ago, separating from animal-like reptiles. The historical path of development of the animal world is called evolution.
During evolution, natural selection occurred - only those animals survived that were able to adapt to environmental conditions. Mammals have evolved in different directions, forming many species. It happened that animals that had a common ancestor at some stage began to live in different conditions and acquired different skills in the struggle for survival. Their appearance was transformed, and changes useful for the survival of the species were consolidated from generation to generation.
Animals whose ancestors looked the same relatively recently began to differ greatly from each other over time. Conversely, species that had different ancestors and went through different evolutionary paths sometimes find themselves in the same conditions and, changing, become similar. Thus, species unrelated to each other acquire common features, and only science can trace their history.
Classification of the animal world
The living nature of the Earth is divided into five kingdoms: bacteria, protozoa, fungi, plants and animals.
Kingdoms, in turn, are divided into types. Exists 10 types animals: sponges, bryozoans, flatworms, roundworms, annelids, coelenterates, arthropods, molluscs, echinoderms and chordates. Chordates are the most progressive type of animals. They are united by the presence of a notochord, the primary skeletal axis.
The most highly developed chordates are grouped into the vertebrate subphylum. Their notochord is transformed into a spine.
Kingdoms
Types are divided into classes. Total exists 5 classes of vertebrates: fish, amphibians, birds, reptiles (reptiles) and mammals (animals).
Mammals are the most highly organized animals of all vertebrates. What all mammals have in common is that they feed their young with milk.
The class of mammals is divided into subclasses: oviparous and viviparous.
Modern two-layer animals
Oviparous mammals reproduce by laying eggs, like reptiles or birds, but feed their young with milk. Viviparous mammals are divided into infraclasses: marsupials and placentals. Marsupials give birth to underdeveloped young, which are carried to term in the mother's brood pouch for a long time. In placentals, the embryo develops in the mother's womb and is born already formed.
Placental mammals have a special organ - the placenta, which carries out the exchange of substances between the maternal body and the embryo during intrauterine development. Marsupials and oviparous animals do not have a placenta.
Types of animals
Classes are divided into squads.
Total exists 20 orders of mammals. In the oviparous subclass there is one order: monotremes, in the marsupial infraclass there is one order: marsupials, in the placental infraclass there are 18 orders: odontates, insectivores, woolly wings, chiropterans, primates, carnivores, pinnipeds, cetaceans, sirenians, proboscideans, hyraxes, aardvarks, artiodactyls, Callopods, lizards, rodents and lagomorphs.
Mammal class
Some scientists distinguish the independent order Tupaya from the order of primates, from the order of insectivores they separate the order Jumpers, and the predators and pinnipeds are combined into one order.
Each order is divided into families, families into genera, and genera into species. In total, about 4,000 species of mammals currently live on earth. Each individual animal is called an individual.
Among plants, these include, first of all, algae and some bryophytes, ferns and flowering plants. Plants of the division classified as flowering are found mainly in fresh water bodies. These include hornwort, lotus, duckweed and others.
Among the marine flowering plants, Zostera can be noted as the most common species of this division of the plant kingdom.
Two-layered animals Coelenterate sponges and others Considered
The fauna of the planet is represented by types of organisms, divided into classes, orders, families and, ultimately, species.
The existing classification distinguishes many different types of animals.
On this page we will look at the simplest and least organized of them.
Protozoa
These are microorganisms that live in various bodies of water - marine and fresh. These include amoebas, radiolarians, etc.

Sponges
This type of animal is represented by multicellular microorganisms. Sponges live in both fresh and marine waters. They lead a sedentary lifestyle. They feed on organic particles extracted from water by filtration.
For a long time, scientists considered sponges to be a kind of transitional form between animals and plants (zoophytes), but later this type was assigned to the animal kingdom.
Coelenterates
A type of animal that is found only in water. An extremely diverse type of living organism. Among them there are marine and freshwater, sessile and free-swimming, solitary and forming numerous colonies.
Examples of coelenterates: hydra (freshwater), jellyfish, corals, various polyps, etc. A distinctive feature of coelenterates is the presence of stinging cells.
Ctenophores
Small mobile animals, similar to jellyfish, but lacking stinging cells.
Tunicates
These animals are similar to sessile coelenterates, i.e.
They lead an attached lifestyle and obtain food by filtering organic matter from the water. They, like ctenophores, do not have stinging cells to kill or paralyze prey.
Flatworms
Marine flatworms resemble long, elegant ribbons.  Reservoirs are inhabited by a small number of types of flatworms: chaetomaxillary worms, nematoleminthes, cephalopods, and tardigrades. Representatives of these types of animals are small, sometimes microscopic organisms.
Reservoirs are inhabited by a small number of types of flatworms: chaetomaxillary worms, nematoleminthes, cephalopods, and tardigrades. Representatives of these types of animals are small, sometimes microscopic organisms.
Nemerteans
Animals that look like worms have a circulatory system.
Nematodes
Annelids
Among all types of worms, these are the most highly organized animals. The largest number of species of annelids live in sea waters. These animals are well known to us from such a common species as the earthworm.
Bryozoans
Aquatic organisms that form colonies.
They live in calcareous shells.
The different types of sessile animals include phoronids, brachiopods, and pogonophora.
Echinoderms, arthropods and others
GENERAL CHARACTERISTICS
Coelenterates are the most poorly organized of the true multicellular animals. The body of coelenterates consists of two layers of cells - ectoderm and endoderm, between which there is a more or less developed non-cellular layer called mesoglea. These animals got their name due to the fact that they have only one cavity, called the intestinal or astral cavity. All coelenterates are aquatic, with the exception of a few species, marine organisms. Their body is built according to the type of radial-axial symmetry.
Despite the simplicity of organization, coelenterates are very diverse in appearance. This depends on two reasons. The first reason is the ability of coelenterates to form colonies. As a rule, an individual colony is very small, and therefore, first of all, attention is involuntarily drawn to the entire colony as a whole. Some colonies look like bushes or small trees. Others look like a pipe cleaner, others resemble fantastic bird feathers. In addition to such delicate flexible colonies, there are massive colonies with a powerful calcareous skeleton. They have the shape of a ball, a goblet, a mushroom, or a prickly Christmas tree. Finally, gentle floating colonies are encountered.
The second reason for the diversity of appearance of coelenterates depends on the fact that in this type of animal an individual has the form of either a polyp or a jellyfish. The body of the polyp is usually cylindrical; at its upper end there is a mouth surrounded by tentacles. Polyps are sedentary or even attached animals; they often form colonies. Jellyfish are solitary swimming, mobile organisms. Their body is shaped like an umbrella with tentacles along the edges. Jellyfish swim with their mouths down.

The presence in the coelenterates of two forms, two states - polypoid and medusoid, the ability of these animals to form colonies of various shapes, as well as their inherent bright coloring make the coelenterates a very diverse group of animals in forms, despite the fact that their internal structure has a single, general plan and structure they are quite simple.
Currently, about 9 thousand species belonging to this type are known. The smallest of them (for example, polyps on hydroid colonies) barely reach 1 mm, the largest, such as cyanea jellyfish(Cyanea arctica), have an umbrella up to 2 m in diameter, and the tentacles of this jellyfish stretch up to 30 m.
Coelenterates are the most ancient of the true multicellular animals. Over the long history of the development of this type, its representatives have managed to adapt very well to a wide variety of living conditions. They populated literally the entire ocean from its surface to the extreme depths; they can be found in the polar regions and in the tropics. Coelenterates settle on a wide variety of soils, some of them are able to withstand significant changes in the salinity of sea water, and some species have even penetrated into fresh waters. Almost everywhere they play a very important role in the formation of communities of marine animals and plants - sea biocenoses. Let's get to know these interesting creatures better.
In order for the reader to more easily understand the systematic position of individual species of coelenterates and to gain a more complete understanding of the composition of this group of animals, a brief type system is given below.
Type Coelenterata
Class Hydrozoa An individual has the form of either a polyp or a jellyfish. The intestinal cavity of polyps is devoid of radial septa. The gonads develop in the ectoderm. About 2,800 species live in the sea, but there are several freshwater forms.
Subclass Hydroids (Hydroidea) Bottom-based, adherent colonies. In some non-colonial species, polyps are able to float at the surface of the water. Within each species, all individuals of the medusoid structure are the same.
Order Leptolida There are individuals of both polypoid and medusoid origin. Mostly marine, very rarely freshwater organisms.
Order Hydrocorallia (Hydrocorallia) The trunk and branches of the colony are calcareous), often colored in a beautiful yellowish, pink or red color. Medusoid individuals are underdeveloped and buried deep in the skeleton. Exclusively marine organisms.
Order Chondrophora A colony consists of a floating polyp and medusoid individuals attached to it. Exclusively marine animals. Previously they were classified as a subclass of siphonophores.
Order Trachylida Exclusively marine hydroids, jellyfish-shaped, no polyps.
Order Alcyonaria Soft corals, skeleton in the form of calcareous needles.
Order Horn corals (Gorgonaria) Skeleton in the form of calcareous needles, usually there is also an axial skeleton of horn-like or calcified organic matter passing through the trunk and branches of the colony.
Order Sea feathers (Penpatularia) A peculiar colony consisting of a large polyp, on the lateral outgrowths of which secondary polyps develop. The base of the colony is embedded in the ground. Some species are able to move.
Subclass Six-rayed corals (Hexacogallia) Colonial and solitary forms. Tentacles without lateral outgrowths; their number is usually equal to or a multiple of six.
Order Actinia (Actiniaria) Solitary, freely moving, non-skeletal polyps living on the surface of the seabed (there are a small number of burrowing species).
Order Madreporaria Corals Mostly colonial, less often solitary (but immobile) corals with a thick external calcareous skeleton.
Order Cork corals (Zoantharia) Solitary or colonial forms growing to the ground. Colonies are creeping.
Order Antipatharia Colonial, branched, accretive corals with an axial skeleton of horn-like substance.
Order Ceriantharia Solitary, non-skeletal polyps living in muddy soil. They build themselves tubes from silt, holding it together with mucous secretions. The polyp is able to move inside the tube.
Order Hydra (Hydrida) Solitary freshwater polyps, do not form jellyfish.
Subclass Siphonophora Floating colonies, which include polypoid and medusoid individuals of various structures. They live exclusively in the sea.
Class Scyphozoa An individual has the appearance of either a small polyp or a large jellyfish, or the animal bears characteristics of both generations. The intestinal cavity of polyps has 4 incomplete radial septa. The gonads develop in the endoderm of jellyfish. About 200 species. Exclusively marine organisms.
Order Coronomedusae (Coronata) Mostly deep-sea jellyfish, the umbrella of which is divided by a constriction into a central disk and a crown. The polyp forms a protective chitinoid tube around itself.
Order Discomedusae The umbrella of jellyfish is solid, there are radial canals. Polyps lack a protective tube.
Order Cubomedusae The umbrella of the jellyfish is solid, but lacks radial canals, the function of which is performed by the far protruding stomach pouches. Polyp without a protective tube.
Order Stauromedusae Peculiar benthic organisms that combine in their structure the characteristics of a jellyfish and a polyp.
Class Coral polyps (Anthozoa) An individual individual has the form of a polyp; jellyfish are not formed. The gonads develop in the endoderm. The intestinal cavity is divided into chambers by radial partitions. About 6000 species. Exclusively marine organisms.
Subclass Eight-rayed corals (Octocorallia) Colonial forms, usually attached to the ground. The polyp has 8 tentacles, on the sides of which there are pinnules.
Order Solar corals (Helioporida) Solid, massive skeleton.
HISTORY OF THE STUDY OF CENTEROCAVITIES
Interest in studying the fauna of marine invertebrates arose in ancient times.
We find the first information about coelenterates in the works of Aristotle. True, in his system they do not represent a single group of the animal kingdom. Individual coelenterates were described by Aristotle in different sections of the system. The main part of the coelenterates was united in the group Cnidae, or cnidarians. Aristotle describes in detail their immobility, notes the presence in the body of cnidarians of only one opening - the mouth, which has a central position and is surrounded by a ring of movable tentacles. The ancient Greek scientist already knew that the tentacles contain special stinging organs that serve to protect against enemies and to grasp prey. Aristotle also made a very interesting statement that coelenterates and, together with them, other simply structured invertebrates leading an attached lifestyle (sponges, ascidians, bryozoans) are organisms in which the nature of animals and plants is unexpectedly mixed. This duality of the nature of coelenterates, in his opinion, was expressed in the fact that they resemble animals in their ability to feed on animal food and perceive external irritations, and in their general structure plan, simplicity of organization and attached way of life they resemble plants. Following Aristotle, another ancient philosopher and naturalist Pliny also saw the dual nature of coelenterates.
The eighteenth and nineteenth centuries were a time of accumulation of new information about the structure and lifestyle of coelenterates. In 1723, an interesting discovery was made by the French ship's doctor Paysonnel. He found that reef-building corals are colonial coelenterates and have a massive calcareous skeleton.
But before its discovery, both navigators and scientists believed that coral polypnyaks belonged to the inorganic world and represented a special calcareous mineral. Paysonnel was able to observe how polyps of reef-forming corals captured small crustaceans swimming past with their tentacles and then ate them.
But the most striking page in the history of the study of coelenterates were the studies of the naturalist Tremblay, a Swiss by birth, who, like many others at that time, was interested in observing the behavior of various small animals - ciliates and “insects” using a microscope. By chance, he discovered freshwater hydras attached to algae - small single hydroid polyps, which he initially mistook for aquatic plants. Tremblay knew nothing about hydras at that time. Yes, this is not surprising, since almost nothing was known about them at all and hydras did not even have a zoological name. Tremblay called the hydra "a polyp with horned arms." The result of long-term observations of hydras was the book “Memoirs of a Freshwater Polyp,” published in 1744. Tremblay described with amazing completeness and accuracy the structure of the hydra, its reproduction, nutrition, methods of movement, etc. But the main achievement of the naturalist should be considered those discovered by experimental research Hydra's ability to regenerate, i.e., self-healing. Regeneration in other organisms was known before, but in previous experiments it was possible to observe the restoration of only individual lost parts of the animal, for example, paws or antennae in crustaceans. In Hydra, Tremblay observed something more - the restoration of a whole organism from its individual parts. This ability was considered by Tremblay and his contemporaries as a special kind of reproduction of animals by cutting it into pieces. Tremblay's work was of great interest. On the one hand, his observations definitively proved the animal nature of the hydra and other zoophytes in general. On the other hand, these studies led to the denial of the opinion about the main difference between animals and plants, which had dominated until that time and was that plants can reproduce by cuttings and layering, but animals cannot.
In the middle of the last century, zoologist Leuckart created an independent type of animals - coelenterates, distinguishing it from the type Zoophita.
COMPARATIVE ANATOMICAL REVIEW OF THE CENTEROCAVITIES
The structure of animals belonging to each of the classes of coelenterates is distinguished by its own characteristics. In this regard, it is more convenient to consider the structure of representatives of each class separately.
Hydroid(Hydrozoa). The body of a hydroid polyp is cylindrical or ovoid in shape, usually equipped with a stalk in its lower part. With the help of a stalk, single polyps attach to the ground, aquatic plants, mollusk shells, i.e., in general, to any underwater objects. In colonial forms, a stalk connects the polyp to the colony. At the upper end of the polyp's body there is a mouth opening surrounded by tentacles. The tentacles can be arranged in regular corollas or without a strict order. The number of tentacles varies among different species. In a giant solitary hydroid polyp branchiocerianthus(Branchiocerianthus imperator), reaching a meter in height, has up to 380 tentacles, and the small one monobrachium(Monobrachium parasiticum) - only one tentacle. More often, each polyp is equipped with 10-30 tentacles, which can be either simple or equipped with a club-shaped thickening at the end. In one species (Cladocoryne) the tentacles even branch.

There are also polyps completely devoid of tentacles. These include freshwater microhydra(Microhydra).
Polyps capture food with their tentacles, and solitary polyps, such as the freshwater hydra, use them when moving.
The internal structure of a hydroid polyp is very simple. It looks like a two-layer bag. The outside of the polyp is covered with a layer of ectoderm cells, and its intestinal cavity is lined with endoderm.

The ectoderm is formed by special epithelial-muscle cells. The body of such a cell has the appearance of a multifaceted prism; the cells are located closely, like a honeycomb. The prismatic part performs the integumentary function. At their inner end, the epithelial cells have a long extension that extends up and down along the body of the polyp. A thin muscle fiber runs inside the process. The same contractile processes extend from endoderm cells, but they are located perpendicular to the processes of ectodermal cells. The combination of fibers of many ectodermal and endodermal cells allows the polyp and its tentacles to stretch and contract.
Between the epithelial-muscular cells there are special stinging cells; they are complex and are very important for all coelenterates as a weapon of attack and defense. A description of the structure and action of these cells will be given below, in a special section on stinging cells and the action of their poison. The ectoderm also contains special nerve cells equipped with long thin processes, which together form a network-like nerve plexus, somewhat denser at the ends of the tentacles and around the mouth. In polyps that can reproduce sexually (for example, hydra), germ cells also develop in the ectoderm. They usually accumulate in the lower or middle part of the body and are called gonads or gonads. Finally, here in the epithelium there are reserve cells (they are called intermediate or interstitial), from which epithelial-muscular, stinging, nerve and germ cells of hydroids develop.
The ectoderm of many types of polyps secretes a thin shell on the outside, consisting of a chitin-like substance. In hydroid polyps, this membrane acts as an exoskeleton, serving as support and protection.
In some species, the membrane around the polyp forms a kind of calyx, into which the polyps can be drawn in case of danger (Fig. 158, 3).

The endoderm consists of glandular and epithelial-muscular cells. Glandular cells produce digestive juice (proteolytic enzyme), which promotes the absorption of proteins. Small animals that enter the gastric cavity of the polyp are digested under the influence of digestive juices and fall apart. Endoderm cells have 2-5 thin flagella, which constantly wriggle and mix the contents of the gastric cavity. Food particles that find themselves near the walls of the gastric cavity are captured by pseudopodia formed on the surface of epithelial muscle cells. Further digestion occurs inside the cell, just as it occurs in single-celled animals. The gastric cavities of individual polyps of the colony communicate with each other, forming a single digestive cavity of the colony.
The endoderm also contains nerve cells, but there are fewer of them than in the ectoderm; stinging cells are completely absent in the endoderm. The presence of nerve cells in the endoderm is characteristic of coelenterates, and partly of echinoderms, but is unusual for all other animals.
Between the layers of ectoderm and endoderm in hydroid polyps there is a thin layer of non-cellular substance - mesoglea.
Hydroid jellyfish are much more complex. Externally, the hydromedusa looks like a transparent disk, umbrella or bell. There are also bizarre forms of jellyfish with ring constrictions in the middle of the body or jellyfish with an almost spherical shape.

An oral proboscis with a mouth at the end hangs from the inner center of the umbrella. The edges of the mouth may be smooth or equipped with four more or less fringed oral lobes. Some hydrojellyfish have small, club-shaped mouth tentacles at the edges of their mouths.
The mouth leads into the stomach, which occupies the entire cavity of the oral proboscis; 4 (occasionally more) radial canals extend from the stomach to the periphery of the umbrella. At the edge of the umbrella they flow into a ring canal. The combination of the stomach and canals is called the gastrovascular system. Along the edge of the hydromedusa umbrella are tentacles and sensory organs.

The tentacles are used for touching and catching prey; they are densely packed with stinging cells. Part of the tentacles can be modified into special sensitive organs, called cones or antennae depending on their shape. In one group of hydromedusae (trachylids), the tentacles are modified into balance organs. Such a tentacle is greatly shortened and sits as if on a thin stalk. A limestone grain, a statolite, is placed at its end. Outside, the tentacle is surrounded by long sensitive hairs (Fig. 143, 4). When the body of the jellyfish tilts, the tentacle, under the influence of gravity, remains hanging vertically and at the same time touches the sensitive hairs, which transmit irritation through the nervous system to the epithelial-muscular cells, which causes contraction of their muscle fibers. There is an assumption that the balance organs (they are called statocysts) serve mainly to ensure that the muscles work rhythmically. The movement of the jellyfish is carried out due to the contraction of muscle fibers at the edge of the umbrella. By pushing water out of the cavity of the umbrella, the jellyfish receives a jet push and moves with the top side of the umbrella forward. Strengthening the reactive ability is achieved due to the presence on the inside of the umbrella of a ring-shaped outgrowth, called a sail, which narrows the exit from the cavity of the umbrella. Each contraction of the circular muscle fibers causes vibrations of the statocysts, which irritate the cells of the nervous system and cause a new contraction. In jellyfish with excised statocysts, the regularity of umbrella contractions is sharply disrupted and their frequency decreases. U hydromedusa from Leptolide group statocysts are absent or arranged in the form of a bubble, inside of which there are several statoliths, and the walls are covered with sensitive cells. Leptolid statocysts have nothing in common with tentacles, but have the same function as trachylid statocysts.
Some hydromedusae have photosensitive organs - eyes, which are always located at the base of the tentacles and are clearly visible due to their dark color. The eye consists of two types of cells - light-sensitive and pigment cells, i.e., those that carry a coloring substance. Due to the presence of pigment cells, light falls on the sensitive cells from only one side. Sensitive cells transmit light stimulation to the nervous system. The simplest ocelli look like spots, while the more complex ones are arranged in the form of pits. In the most complex ocelli, the cavity of the fossa is filled with a transparent substance that acts as a lens (Fig. 143, B).

Due to the freely mobile lifestyle of hydromedusae, their nervous system is much more developed than that of hydropolyps. Although the plexus also has the appearance of a network, at the edge of the umbrella the nerve cells accumulate very densely and form two rings. One of them (external) is sensitive, the other (internal) motor.
The sensitive ring passes near the statocysts, ocelli and bases of the tentacles and perceives the irritations received from them. The motor ring lies at the base of the velum, where a large number of circular muscle fibers are concentrated, which are innervated from the motor nerve ring.
Jellyfish are dioecious; their gonads are located either in the ectoderm of the oral proboscis or in the ectoderm of the umbrella under the radial canals. Here they are closest to the nutrients necessary for the development of reproductive products. The structure of the cells of the ectoderm and endoderm of jellyfish is the same as that of polyps, and therefore does not require additional description, but the mesoglea of jellyfish is much more developed. It is rich in water and has a gelatinous nature, due to which hydromedusae are very transparent; many, even quite large, jellyfish are difficult to see in the water. The mesoglea in the umbrella is especially strongly developed.
The general characteristics given above apply only to one subclass of hydroids(Hydroidea), but in hydroid class(Hydrozoa) also includes a very peculiar subclass siphonophore(Siphonophora). Siphonophores live only in the sea. They are similar to colonies of hydroid polyps, which, as a whole, have switched to a pelagic existence.
Colonies of siphonophores are characterized to the greatest extent by the phenomenon of polymorphism, equal to which can be found only among ants and termites. A siphonophore colony includes individuals that have a special structure and perform various functions. Some of them perform the functions of movement, others - nutrition, others - excretion, others - reproduction, and others - protection. Siphonophores are one of the most amazing and most beautiful creatures of the sea element.
Scyphoid(Scyphozoa). Polyps belonging to this class are very small, only a few millimeters in height. As a rule, they do not form colonies and in most cases lack a skeletal membrane. The body of a scyphoid polyp (also called a scyphistoma) is divided into a calyx and a stalk.

In the upper part of the body, along the edge of the calyx, the scyphistoma is equipped with a corolla of tentacles, between which a quadrangular oral opening is placed at the oral end. The gastric cavity of the scyphistoma is divided by four incomplete internal partitions (septa, tenioli) into a central part and 4 peripheral chambers. In the upper part, the tenioli are carried along a small hole, through which neighboring chambers communicate with each other. A conical cavity, a funnel, protrudes into each teniole from the side of the oral cone. A muscle cord stretches down from the bottom of the funnel. The structure of the ectoderm and endoderm of scyphistoma in general resembles that of hydroid polyps.
Externally scyphoid jellyfish have significant similarities with hydroids, but significantly exceed them in size. The bell shape, coloring and fringed edges, mouth lobes and numerous tentacles of scyphoid jellyfish make them the most beautiful organisms living in the sea. The body of the scyphomedusa has the shape of a disk, umbrella or bell. In the center of the lower side there is a mouth opening, surrounded by 4 oral lobes. In the spaces between the oral lobes, many scyphojellyfish have invaginations, the so-called subgenital fossae. They are located under the gonads and apparently serve to facilitate oxygen access to developing reproductive products.

The edge of the scyphomedusa umbrella has a rosette-shaped shape, as it is divided into 8 or 16 marginal lobes, tentacles are attached to their lower side, and between them there are rhopalia - small, modified tentacles carrying sensory organs - eyes and statocysts. The number of tentacles varies among different groups of scyphojellyfish and ranges from 4 to several hundred. U rooted jellyfish tentacles are missing.
The gastric cavity of scyphoid jellyfish is quite complex. The mouth leads into a short tube-shaped pharynx, which opens into the stomach, which has 4 side pockets. Inside the pouches, the walls of the stomach form numerous long endodermal projections, the so-called gastric filaments. They secrete digestive juices into the intestinal cavity. Radial canals extend from the stomach to the periphery of the umbrella, the number of which is usually equal to or a multiple of 8.
In a number of species, the radial canals branch or even form a network. At the edge of the umbrella, the radial canals flow into the annular canal, but sometimes the latter is absent.

The nervous system of scyphojellyfish is a plexus of nerve cells. Along the edge of the bell there is a nerve ring, and near the ropalia nerve elements are concentrated, forming loose clusters here similar to ganglia. Rhopalium is a small underdeveloped modified tentacle, bearing in its end part a group of calcareous bodies - statoliths. Touching the tip of the rhopalium to the sensitive cells of the marginal blade causes irritation, which is transmitted through the nearest cluster of nerve cells to the muscles of the umbrella, causing its contraction. In this way the rate of pulsation of the umbrella is regulated. In specimens with removed rhopalia, the regularity of contractions is disrupted. The rhopalium, as well as a hydrostatic organ, allows the jellyfish to orient its body with its mouth opening downward.
In many scyphoid jellyfish, light-sensitive organs are also located on the rhopalia - eye spots and more complex ocelli, reminiscent in structure of the hydromedusa ocelli described above.
Scyphoid jellyfish are dioecious; their gonads develop in the endoderm and are located in the pouches of the stomach. Reproductive products are expelled through the mouth.
The mesoglea of scyphoid jellyfish, like that of hydromedusae, is very developed.
Coral polyps(Anthozoa). The body of a coral polyp is usually cylindrical in shape and is not divided into a trunk and a leg. In colonial forms, the lower end of the polyp body is attached to the colony, and in single polyps it is equipped with an attachment sole. The tentacles of coral polyps are located in one or several closely spaced corollas. There are two large groups of coral polyps: eight-rayed(Octocorallia) and six-rayed(Nehacorallia). The former always have 8 tentacles, and they are equipped at the edges with small outgrowths - pinnules; in the latter, the number of tentacles is usually quite large and, as a rule, a multiple of six. The tentacles of six-rayed corals are smooth and without kicks.

The upper part of the polyp, between the tentacles, is called the oral disc. In its middle there is a slit-like mouth opening. The mouth leads into the pharynx, lined with ectoderm. One of the parts of the oral fissure and the pharynx descending from it is called the siphonoglyph. The ectoderm of the siphonoglyph is covered with epithelial cells with very large cilia, which are in continuous movement and drive water into the intestinal cavity of the polyp. The intestinal cavity of a coral polyp is divided into chambers by longitudinal endodermal septa (septa). In the upper part of the body of the polyp, the septa grow with one edge to the body wall and the other to the pharynx. In the lower part of the polyp, below the pharynx, the septa are attached only to the body wall, as a result of which the central part of the gastric cavity - the stomach - remains undivided. The number of septa corresponds to the number of tentacles. Along each septum, along one of its sides, there is a muscular ridge. The free edges of the septa are thickened and are called mesenteric filaments. Two of these filaments, located on a pair of adjacent septa opposing the siphonoglyph, are covered with special cells bearing long cilia. The cilia are in constant motion and drive water out of the gastric cavity. The joint work of the ciliated epithelium of these two mesenteric filaments and the siphonoglyph ensures a constant change of water in the gastric cavity. Thanks to them, fresh, oxygen-rich water constantly enters the intestinal cavity. Species that feed on tiny planktonic organisms also receive food. The remaining mesenteric filaments play an important role in digestion, as they are formed by glandular endodermal cells that secrete digestive juices.
The gonads develop in the endoderm of the septa. In coral polyps, the skeleton plays a very important role.
Eight-rayed corals have a skeleton consisting of individual calcareous needles - spicules located in the mesoglea. Sometimes the spicules are connected to each other, merging or being united by an organic horn-like substance.
Among the six-rayed corals there are non-skeletal forms, such as sea anemones. More often, however, they have a skeleton, and it can be either internal - in the form of a rod of horn-like substance, or external - calcareous.

The skeleton of representatives of the madreporidae group reaches especially great complexity. It is secreted by the ectoderm of the polyps and at first has the appearance of a plate or low cup in which the polyp itself sits. Next, the skeleton begins to grow, radial ribs appear on it, corresponding to the septa of the polyp. Soon the polyp appears as if impaled on a skeletal base, which protrudes deeply into its body from below, although it is delimited throughout by ectoderm. The skeleton of madrepore corals is very strongly developed: soft tissues cover it in the form of a thin film.
The skeleton of coelenterates plays the role of a support system, and together with the stinging apparatus, it represents a powerful defense against enemies, which contributed to their existence over long geological periods.
Coral polyps are characterized only by the polypoid state; they do not form jellyfish.
STINGING CELLS OF THE CENTERAVENTS AND THE EFFECT OF THEIR POISON
The most characteristic feature of all coelenterates is the presence of special stinging, or nettle, cells. These cells develop from undifferentiated intermediate ectoderm cells. Each stinging cell contains an oval or oblong chitinoid capsule. The wall of the outer end of the capsule protrudes into it and has the appearance of a thin spirally twisted tube called a stinging filament. The capsule cavity is filled with a toxic liquid.

On the outer surface of the stinging cell there is a sensitive hair - cnidocil. Touching a hair causes instant irritation of the cell, manifested in a rapid, shot-like inversion of the stinging thread. Depending on the details of the structure and the method of influencing the enemy or victim, several types of stinging capsules are distinguished. Some of the stinging capsules have a long thread equipped with spines. When such a capsule is “shot,” the thread is pierced into the victim’s body, and the contents of the capsule are poured through its cavity, causing general or local poisoning. Other capsules have a short thread, devoid of spines. Such threads only entangle the victim. Finally, there are sticky threads that keep the prey glued. They can also serve for temporary attachment of the stinging cell carriers themselves during movement.
Stinging cells are located over the entire surface of the body of coelenterates, but their largest accumulations are observed on the tentacles and around the mouth opening, i.e., in the places where they are most needed. After the “shot,” the stinging cell dies and a new stinging cell develops in its place or nearby.
It is very interesting that stinging capsules can act even after the death of the animal. Thus, touching dead, beached jellyfish can cause quite severe redness of the skin, accompanied by a burning sensation.
Stinging cells, especially those containing a poisonous liquid in capsules, are a formidable weapon that is used by coelenterates for both defense and attack. There is no doubt that the prosperity of these lowly organized animals is largely due to the presence of stinging cells. Small animals, in contact with coelenterates, become stuck, entangled and pierced by stinging threads, and poison enters their body, causing paralysis and death. After this, the victim is carried by the tentacles to the mouth opening and swallowed. Even fairly large animals are often affected by the poison of the stinging capsules, which also causes burning pain. The strength of the effect of the poison of different species of coelenterates on humans is not the same: some of them are completely harmless, others pose a serious danger.
After touching our Black Sea sea anemones and to cyanea jellyfish, living in the Arctic and Far Eastern seas, a slight burning sensation is felt, especially where the skin is more delicate, for example, between the fingers. It “burns” a little more cornermouth- a whitish jellyfish with a purple umbrella edge, common in the Black Sea. Nevertheless, these unpleasant sensations, similar to a slight nettle burn, quickly pass without leaving a trace. However, there are many much more dangerous coelenterates in the sea, touching which can lead to serious illness. Thus, many sea anemones, including those living in the Sea of Japan, cause not only “burns”, but also long-term swelling of the hand of a person carelessly touching them.

However, the most unpleasant consequences are not “burns” and tumors, but general poisoning of the body with the poison of stinging cells. The beautiful siphonophore has long been notorious among sailors sailing in tropical seas. physalia. The physalia is equipped with a large, up to 20 cm long, swim bladder, rising above the surface of the water, from which the longest (up to 30 m) hunting tentacles, equipped with numerous stinging cells, hang down. Physalia is very brightly colored. The bubble is cast in blue, violet and purple colors, all the appendages hanging down are ultramarine. For its bright color, physalia also received the name Portuguese warship: In the Middle Ages, the Portuguese liked to paint their warships colorfully. Physalia floating on the surface of the sea, due to its bright color and significant size, is visible from afar, and swimmers are always careful not to come into contact with it, as they can get a very strong “burn”, causing severe pain. A person affected by physalia, even if he is a good swimmer, has difficulty staying on the water. Soon after this, a general severe illness with fever may occur, lasting several days. Physalia are distributed exclusively in tropical seas, and very rarely individual specimens are brought into warmer areas of the temperate zone.
In recent years, reports of serious poisonings caused by large scyphoid jellyfish have begun to appear in a number of medical and biological journals. chirodropus(Chirodropus) from box jellyfish squad.
It is also an inhabitant of tropical seas. The height of the jellyfish bell reaches 10-12 cm; on the edge of the umbrella there are 4 tentacles, each of which is branched and has 9-12 ends. The jellyfish is translucent and difficult to see in the water. Of course, the poisonous properties of this jellyfish did not appear in recent years, but before they simply did not pay attention to it due to the general poor knowledge of the coelenterate tropical seas.
For the first time, attention was paid to the toxicity of this jellyfish during the Second World War in Australia, where a significant number of Europeans were evacuated. Several cases of mysterious deaths of people while swimming have become known, and no traces of shark teeth or other obvious signs of damage were found on the bodies of the victims. For a long time, the mystery remained unsolved, but then it was established that the cause of death was a jellyfish. The person who received the “burn”, barely having time to scream from the piercing pain, choked and drowned. It was noted that among the injured or dead were mainly new arrivals. Local people, especially indigenous Australians, could swim without fear. Apparently, they were immune to the poison of this jellyfish.
In the fauna of our Far Eastern seas there is also one jellyfish that causes serious diseases upon contact with it. Locals call this jellyfish "cross" for the cross-shaped arrangement of four dark radial canals, along which four also dark-colored gonads stretch. The umbrella of the jellyfish is transparent, faint yellowish-green in color. The size of the jellyfish is small: the umbrella of some specimens reaches 25 mm in diameter, but usually they are much smaller, only 15-18 mm. At the edge of the umbrella of the cross (scientific name - Gonionemus vertens) there are up to 80 tentacles that can strongly stretch and contract. The tentacles are densely seated with stinging cells, which are arranged in belts. In the middle of the length of the tentacle there is a small suction cup, with the help of which the jellyfish attaches to various underwater objects.
Crossfishes live in the Sea of Japan and near the Kuril Islands. They usually stay in shallow water. Their favorite places are thickets of sea grass Zostera. Here they swim and hang on blades of grass, attached with their suckers. Sometimes they are found in clean water, but usually not far from zoster thickets. During rains, when sea water off the coast is significantly desalinated, jellyfish die. In rainy years there are almost no of them, but by the end of the dry summer, crosses appear in droves.
Although crossfishes can swim freely, they usually prefer to lie in wait for prey by attaching themselves to an object. Therefore, when one of the tentacles of the cross accidentally touches the body of a bathing person, the jellyfish rushes in this direction and tries to attach itself using suction cups and stinging capsules. At this moment, the bather feels a strong “burn”; after a few minutes, the skin at the site of the tentacle’s contact turns red and becomes blistered. If you feel a “burn”, you need to immediately get out of the water. Within 10-30 minutes, general weakness sets in, pain in the lower back appears, breathing becomes difficult, arms and legs go numb. It’s good if the shore is close, otherwise you might drown. The affected person should be placed comfortably and a doctor should be called immediately. Subcutaneous injections of adrenaline and ephedrine are used for treatment; in the most severe cases, artificial respiration is used. The disease lasts 4-5 days, but even after this period, people affected by the small jellyfish still cannot fully recover for a long time.
Repeated burns are especially dangerous. It has been established that the poison of the cross not only does not develop immunity, but, on the contrary, makes the body hypersensitive even to small doses of the same poison. This phenomenon is known medically as anaphyloxia.
It is quite difficult to protect yourself from a cross. In places where a lot of people usually swim, to combat the crossworm, they mow down the zoster, fence the bathing areas with fine mesh, and catch the crossfish with special nets.
It is interesting to note that such poisonous properties are possessed by crossfish that live only in the Pacific Ocean. A very close form, belonging to the same species, but to a different subspecies, living on the American and European coasts of the Atlantic Ocean, is completely harmless.
Along with animals that are easily affected by stinging cells, there are those on which the venom of coelenterates has no effect at all. Usually these animals settle in a community with coelenterates, which serve as reliable protection for them, and sometimes such cohabitation is mutually beneficial for both.
LIFE CYCLE OF CELINARITIES, ALTERNATION OF GENERATIONS
Of the modern coelenterates, the life cycle is completed most simply coral polyps. The fertilized egg begins to fragment. First, it divides in two, then each of the resulting cells, in turn, also divides, and so on. As a result, a large number of small cells are formed, arranged in one layer and looking like a small hollow ball. Following this, some of the cells begin to sink inside, resulting in a two-layer embryo. The endoderm is subsequently formed from its inner layer, and the ectoderm of the future polyp is formed from the outer layer. The ectoderm is covered with numerous small cilia, with the help of which the embryo gains the ability to swim; from this moment it turns into a larva called a planula. The planula is unable to feed or reproduce. It swims in the water column for some time, then sits on the bottom, attaching itself to it with its front end. Soon after this, a mouth opening breaks through at the posterior (now upper) end of the planula and a corolla of tentacles is formed. This is how the first polyp appears. In colonial forms, this polyp soon buds on other polyps, which in turn follow, etc. A colony arises. Once the colony reaches a certain stage of development, the polyps that make it up begin to reproduce also sexually, forming eggs. This completes the cycle.
Otherwise, the life cycle proceeds hydroid And scyphoid. The crushing of the egg and the development of the planula occur in them in the same way as in coral polyps; in a similar way, the first polyp - the founder of the colony - appears, and then the entire colony (in scyphoid colonies, colonies are usually not formed). However, hydroid and scyphoid polyps are completely devoid of the ability to reproduce sexually, but they bud or separate not only similar polyps, but jellyfish that are completely different from them. Jellyfish are sexual individuals. The formation of jellyfish in hydroid and scyphoid jellyfish occurs differently. In hydropolyps, jellyfish arise from the buds. At first, such a kidney looks similar to a regular kidney from which a polyp develops, but then differences appear. The medusoid bud greatly increases in size, becomes translucent, 4 radially located canals and an oral proboscis extending from the place of their crossing appear inside it. Now the young jellyfish looks like a small bell or an umbrella. Soon she breaks away from the colony of polyps and swims away. Jellyfish feed on their own and increase in size, then they develop gonads. The eggs and sperm are released directly into the seawater, where fertilization of the eggs occurs. From the eggs a larva emerges - a planula. Figure 149 shows a typical life cycle using the example of a hydroid from sort of Korine(Sogupe sarsii).

In scyphoids, as a rule, polyps (they are called scyphistomas) do not form colonies: the polyps that arise as a result of budding are separated from the mother scyphistoma and lead an independent lifestyle. Soon, each such polyp undergoes serious changes: its tentacles shorten, and a series of ring-shaped constrictions appear on the body, which become deeper and deeper, as shown in Figure 150. Then, starting from the upper end, disc-shaped jellyfish separate from the polyp (at this stage they called ethers), which turn their mouth side down and float away. Ethers feed, grow, and develop into large, mature scyphoid jellyfish that reproduce exclusively sexually.

Thus, using the example of hydroids and scyphoids, we have the opportunity to get acquainted with a very peculiar phenomenon called alternation of generations. Indeed, in the life cycle of these two groups of coelenterates, there is a regular replacement of the benthic, sessile, often colonial polypoid generation, reproducing vegetatively, with the free-swimming, solitary, medusoid generation, reproducing sexually. Different generations lead different lifestyles and are structured differently, therefore, before the discovery of the phenomenon of alternation of generations, they were considered independent groups of animals.
The alternation of generations is very important for the life of hydroids and scyphoids: the attached lifestyle of polyps prevents their settlement, and jellyfish, having left the colony, can be carried far away by sea currents and scatter eggs over a very large space, populating more and more new areas. The alternation of differently arranged generations is quite common in the animal world. In addition to coelenterates, it is found in some flat, round and annelid worms, in a number of crustaceans and even in lower chordates.
It was among representatives of chordates - salps - that the famous zoologist and poet Shamissov discovered the alternation of generations in 1819. This discovery led to the search for new cases of alternation of generations. In 1841, M. Sars described the alternation of generations in two scyphoid jellyfish - aurelia(Aurelia) and cyanea(Suanea), and a year later Steenstrup established that hydroids also develop with alternating generations.
The research of Chamisso, Sars and Steenstrup was a significant event for zoologists of the last century, thanks to them it was necessary to revise many old ideas about biology and systematics.
The method of alternation of generations described above in coelenterates is called metagenesis, and animals with this type of life cycle are called metagenetic. Metagenesis is characterized by the correct alternation of the sexual generation with a generation that reproduces vegetatively, by division or budding.
One should not think that the life cycle of all hydroid and scyphoid species follows strictly according to the scheme with which we have already become familiar. In most cases, there are more or less noticeable deviations from this typical manifestation of metagenesis, depending on a number of reasons.
The main reason causing a violation of proper metagenesis should be considered the difference in environmental conditions in which polyps and jellyfish live. In fact, the benthic, immobile lifestyle of polyps inevitably causes them to develop a number of adaptations that meet the conditions of their existence. Since polyps sit motionless on the bottom, they do not need special sensory organs that allow them to recognize from a distance the approach of an enemy or prey: they still cannot get closer to the prey or get away from enemies. Therefore, the polyps retained only the sense of touch, allowing them to gropingly catch prey that touched the spaced tentacles. Another way to protect polyps is to form an exoskeleton. The stronger and heavier the skeleton, the more reliably it protects the polyps and the better it holds the entire colony on the seabed. The above features of polyps are not characteristic of coelenterates alone - they are produced in many attached aquatic organisms. A common property of most sessile animals is also the formation of colonies. It is very typical for coelenterate polyps and siphonophores. The formation of colonies is often associated with the division of functions among its members. This is especially pronounced in hydroid polyps. Only in a very few species are all polyps arranged in the same way and each of them can bud off jellyfish. Much more often, some polyps, equipped with tentacles and a mouth, catch prey for the colony, but are not able to reproduce, while others lack tentacles and a mouth, but are adapted exclusively to budding jellyfish. This is the case with the widespread hydroid obelia(Obelia geniculata). In such species, the correctness of metagenesis is disrupted, since some of the polyps (nursing individuals) do not produce sexual offspring.
The more complex a colony becomes, the more important it becomes in the life cycle. In this case, the role of jellyfish decreases. A large colony can feed not only polyps, but also jellyfish. Therefore, jellyfish do not break away from the colony for a long time and leave it only when their reproductive products mature. Finally, there are many hydroid species whose colony is so complicated that jellyfish stop detaching altogether and become attached organisms like polyps. At the same time, the characteristic features of a jellyfish - an umbrella, eyes, channels of the gastrovascular system - are gradually lost and the jellyfish becomes like a sac filled with eggs. Freshwater hydras do not form jellyfish at all.
But let's return to typical metagenesis and follow free-swimming jellyfish. Their lifestyle is very different from that of polyps. Jellyfish are mobile animals, and therefore they develop adaptations for movement and orientation in space. First of all, this concerns the development of the umbrella and muscles, as well as sensory organs. In some species, these adaptations are so well developed that jellyfish can swim far from the places where they began their independent life, having separated from the polyp. They often end up in places where there are no suitable conditions for the development of the polypoid generation, for example, in the open sea, over great depths. The planulae of such jellyfish, not finding a suitable place to attach, die. But this is not the case with all types of hydroid and scyphoid jellyfish. Some of them have adapted to life precisely in the surface layers of water in the open sea, but at the same time their life cycle has changed.
At the hydromedusa bougainvillea(Bougainvillia platygaster) the eggs are not thrown into the water, but develop in the female’s gonad, and here the formation of small polyps occurs, which bud new jellyfish. This case is not the only one; the development of polyps directly on the gonad of a jellyfish is also typical for one species campanularia(Campanularia maccrady), and for some other jellyfish. Although these species have a polypoid generation, it is no longer bottom-based and does not form large colonies. In some species, the suppression of the polypoid stage went even further and polyps completely disappeared from the life cycle. From the planula of such jellyfish, it is not polyps that arise, but the same jellyfish. This is how the life cycle of a large group of hydroid trachylid jellyfish proceeds, and of the scyphoid species genus Pelagia(Pelagia) living in the open sea.
As can be seen from the above examples, the metagenetic life cycle is very plastic. As long as it is beneficial for the prosperity of the species, it is preserved, but as soon as conditions change and one generation gains some advantage, the other generation is suppressed and may completely disappear.
FORMATION AND STRUCTURE OF CENTEROCENTARY COLONIES
Many invertebrate animals have a special type of reproduction, in which a tubercle in the form of an overgrown group of cells is formed on the surface of the body of the mother’s body. This tubercle increases in size and changes its shape until it becomes a smaller copy of the mother's body. This type of reproduction is called budding. Buds can form on both the internal and external surfaces of the body. External budding is much more widespread in nature and serves as a very common method of reproduction in coelenterates. In single polyps, the kidney, which has reached a certain stage of development, becomes detached and begins to exist independently. This is how the common freshwater hydra reproduces. On a saccular-cylindrical body hydra an outgrowth is formed, in the formation of which both the ectodermal and endodermal layers take part. The outgrowth, increasing in size, takes on a cylindrical shape, at its upper end a corolla of tentacles is formed and a mouth opening breaks through. Thus, a daughter hydra is formed on the body of the hydra, already capable of an independent lifestyle, but having a slightly smaller size. Often, however, on the body of such a daughter hydra, which has not yet had time to separate from the mother, a new bud in turn forms. The budding process can go even further, and then a rather complex complex of several interconnected hydras with a gastric cavity common to all of them arises.
The described hydra complex has great integrity and physiological independence. On the other hand, this complex whole is obviously divisible, since each of the young hydras is a potentially independent organism. And indeed, when a certain moment comes, individual polyps begin to detach one by one and move on to an independent lifestyle. The complex under consideration is called a temporary colony.
Much more often, however, in coelenterates the formation of real, or permanent, colonies is observed. Coelenterate colonies are surprisingly diverse. Among them there may be creeping and tree-like, feathery and brush-shaped, graceful and thin colonies and colonies with a massive calcareous skeleton. The color of the colonies is also varied.
The shape of the colonies depends primarily on the nature of the skeleton and the degree of integration of the colony. All polypoid colonies (except sea feathers) are firmly attached to the ground or to some solid objects lying on the ground. Colonies are attached using an expanded base. In hydroids, this basis is the interweaving of creeping filaments, or hydrorhiza. It forms immediately after the hydroid larva settles on the ground or other substrate. The first polyps of the colony bud from the hydrorhiza. In coral polyps, the formation of special base filaments is not observed. Their colonies are attached with the help of a fleshy soft sole or with the help of an expanded calcareous or horny basal plate. Among the deep sea spiny corals(Antipatharia) there is a species, Bathypathes patula, widespread in the ocean, represented by two ecological forms. One of them has a base in the form of a basal plate, the other has a hook-shaped base. For this reason, both forms were previously considered even as two independent species. The formation of one or another form of the base of these corals is determined by the substrate on which their larva settles. Greater variability in the shape of the base of colonies can also be found among other coelenterates. For example, the soft coral Eunephthya, which usually settles on rocks and rocks in the shallow horizons of the northern seas, has a fleshy, wide sole. In the same coral, developing on liquid silty soils, the base grows into several bubbly cavities that capture portions of the soil. These cavities look like bags filled with sand and serve as ballast for the colony, which helps it maintain an upright position (Fig. 151).

The attachment plate is not formed only in representatives of the order sea feathers. In these animals, a muscular expansion is formed at the lower end of the stem, with the help of which the colony penetrates into the ground. Sea feathers are the only colonial coral polyps that can move independently from one place to another. Movement along the ground is carried out by wave-like contractions of the expanded base of the colony.
Creeping and crust-like colonies are the most simple in structure. In such colonies, individual polyps arise directly from the reticulate, filamentous hydrorhiza (in hydroids) or from the membrane-like soft plate covering the substrate (in soft eight-rayed corals or zoantharians). In these cases, neither the filaments of hydrorhiza nor the membranous plate belong to any one polyp, but represent the common body of the entire colony - the coenosarc. The most striking examples of such colonies are the hydroids Hydractinia or Perigonimus and the soft eight-rayed corals Clavularia. Much more often, however, coelenterates form tree-like colonies. Then, from the basal plate or hydrorhiza, a branch-bearing trunk emerges, consisting of one or more interconnected tubes.

The type of branching, and therefore the overall shape of the colony, depends on the location of the buds or growth zones. In some hydroids, most often in those whose polyps are not protected by a calyx, and in sea feathers, the central trunk of the colony is formed by increasing the length of the stalk of the very first polyp larva after settling. He always remains at the top of the colony. Directly below it is the zone of growth and budding of new, secondary polyps. In turn, on the legs of secondary polyps, directly below them there is also a zone of growth and budding. Due to these zones, branches of colonies and polyps covering them are formed (Fig. 152). The branching of such monopodial, as they are called, colonies can be random in the simplest cases, then branches and polyps extend from the trunk in all directions.
This is how the colonies of many hydroids and the most simply organized colonies of sea feathers branch. In more complex colonies of sea feathers, secondary polyps extend from the trunk in one or more planes and at an equal distance from each other or from the so-called “leaves,” i.e., lateral outgrowths, also located in regular rows. This correct arrangement of secondary polyps and “leaves” of the colonies is ensured by the fact that budding occurs only in one plane.
Some more highly organized hydroids and coral polyps have a different type of colony branching. Their budding zone is also located below the calyx of the polyp, but the stalk of the latter has limited growth. Therefore, after the first polyp has formed, the leg of the next polyp begins to form under its base, growing upward and to the side. Sometimes several legs appear simultaneously in the growth zone, giving rise to several branches. With this type of branching, called sympodial, the oldest polyps remain at the base of the colonies and their branches. Sympodial colonies are also characterized by a constant fracture of the longitudinal axis of the trunk and branches at the origin of new polyps (Fig. 152).

This is how many hydroids grow and branch, the polyps of which are protected by cups, and some coral polyps. But the highest type of branching of colonies of hydroids and coral polyps is one in which the zone of growth and budding is not below the primary polyp, but at the top of the outgrowth of the hydrorhiza or basement membrane. In this case, the function of growth and budding passes to the common body of the colony, or coenosarcus. The main trunk grows upward, gradually budding polyps from itself or forming new coenosarcal outgrowths and lateral branches. It is very characteristic of hydroid colonies with coenosarcal growth that individual polyps are devoid of legs and are more or less immersed in the thickness of the branches of the colony. We find coenosarcal growth of colonies, except for hydroids, in most coral polyps.
In more highly organized colonies, branches and polyps, as a rule, are ordered in their arrangement; in some hydroids, the branches are located in groups, within which they diverge radially in all directions and lie in the same plane, perpendicular to the trunk. Such colonies are called whorled. A very common type of branching is in which lateral branches extend from opposite sides of the trunk. In this case, the bases of the branches can be exactly opposite each other, or the branches of one row can be shifted upward from the bases of the branches of the opposite row. In this case, hydroids form graceful feathery colonies, and in some gorgonian corals and antipataria, which have a stronger calcareous or horny skeleton, fan-shaped and plate-like colonies. In this case, neighboring branches often merge with each other, and then these usually very brightly colored fan-shaped colonies take the form of a lattice plate. Sometimes such plates bend in waves, resembling the folds of a curtain. There are colonies whose curved plates form additional lateral plates. Among the eight-rayed coral polyps, very curious colonies form the so-called coral organ(Tubipora). The skeleton of the organ consists of long calcareous tubes rising from the basal plate, formed from fused calcareous skeletal bodies. These tubes, reminiscent of organ tubes, are also connected to each other at different levels by transverse plates. Both the tubes and the plates connecting them are painted a beautiful crimson-red color.

With the strong development of the calcareous skeleton, individual branches merge with each other, and the entire colony then takes the form of a massive monolith. Such colonies are formed among a group of hydrocorals, in which the usual chitinoid skeleton is impregnated with lime and significantly thickened, and among six-rayed reef-forming corals. Powerful coral polypnyaks form reefs and even entire coral islands. It’s not for nothing that just 200 years ago it was believed that coral reefs belong to inorganic nature, representing special mineral nodules! In the most massive madrenore corals, the skeletal cups of the polyps, coming closer together, merge with each other, and then intricately writhing lines with beautiful transverse striations appear on the surface of the polypnyak.
The structure of polyps and the nature of their location on the branches of the colonies are closely related to the shape of the colony itself, as well as to nutrition. All coelenterates are carnivores. They feed on planktonic organisms, small invertebrates that lead a benthic lifestyle, animal remains suspended in water, and even small fish and their larvae. Most colonial coelenterates feed on small planktonic animals. In the most primitive colonies (in the form of a plate creeping on the ground), the polyps acquire an elongated cylindrical shape or rise high on a thin stalk. This elevated position of the polyps above the plate makes it easier for them to catch prey. On the other hand, the long stalk or elongated shape of the polyp makes them more vulnerable to those predators that may feed on them. Therefore, the evolution of colonial forms went in the direction of acquiring tree-like trunks and at the same time reducing the length of the stem. Nevertheless, many more simply organized hydroids with upward-rising trunks retain thin long legs of polyps. Many creeping colonies find a more convenient way to successfully catch bottom waters by settling on the trunks of other, more highly organized colonies of coelenterates. In this case, the need to form legs or their own erect trunks in such coelenterates disappears, and distance from the ground is achieved through the use of trunks and branches of other colonies. Epiphytic colonies are found among hydroids and zoantharians. In the most highly organized hydroids, which form strong tree-like or simple colonies, the legs of the polyps disappear completely, and the calyxes of the polyps either grow on one side to the trunk, or are completely immersed in the thickness of the branch or trunk. Polyps of eight-rayed corals are either able to be retracted into the body of the colony or are protected by special skeletal formations.
The success of catching polyps in the water depends not only on how high they are raised above the ground, but also on the general shape of the colony. Let us imagine a densely and randomly overgrown hydroid bush and compare the position of the polyps sitting at the ends of the branches and in the thicket itself. Naturally, those polyps that are located at the ends of the branches are in more favorable conditions and are more likely to catch prey than those that are located in the thicket of the bush. Therefore, further ordering of the branches, which we have already discussed, contributes to better nutrition of the colonies. But this alone is also not enough. The ordering of branches is correlated with the ordering of the position of individual polyps on them. If the polyps are too crowded, they will interfere with each other when catching prey. Catching prey should be much more successful when polyps are arranged in longitudinal rows and in a checkerboard pattern. Indeed, most coelenterate colonies have a two-row arrangement of polyps. In many hydroids, in addition, there is often a position of the polyps in which the openings of their cups are alternately directed in different directions. By doubling the rows, colonies with four or more rows of polyps could arise. In the most highly organized colonies, a multirow arrangement of polyps is observed. In such rows, the polyps are arranged in a checkerboard pattern. However, there are cases where the arrangement of polyps in one row gives the colony significantly greater advantages than a multi-row arrangement. In particular, this position of polyps was found in deep-sea colonies antipatarians(Bathypathes lyra). Its structure will be described in the appropriate section. Among the colonies of coral polyps, we also find those whose polyps are located in complete disorder. However, in this case, there is a distinct difference in the height of the tentacle corollas, provided by the different lengths of the trapping polyps. At the same time, none of the polyps, even if they have fully expanded their tentacles, interfere with their neighbor - on the same floor the polyps sit at a sufficient distance from each other.
So, a study of the structure of colonies of hydroid and coral polyps shows that in nature there is a clearly expressed desire to organize branches and polyps and increase their number. For some groups of coelenterates, a certain relationship was found between the number of individuals in the colony and the size of individual polyps. As a rule, the more individuals in a colony, the smaller their size.
Colonies of the same species living in polar regions have fewer branches and polyps than colonies living in warm waters. The size of the polyps in the former is noticeably larger than in the latter. The increase in size occurs especially clearly in species that conquer great depths of the ocean. Using many genera of sea feathers, which have their representatives in both shallow and deep-water zones of the oceans, it is possible to construct vivid morphological series that illustrate this process. For example, in a predominantly shallow-water species from the genus Kophobelemnon - K. stelliberum, the height of the colonies reaches 300-350 mm, several dozen polyps can be counted on the trunk. When straightened, they reach a length of 6-10 mm. In a similar but deeper-sea species, K. polyflorum, which lives at a depth of 1000-3500 m, colonies reach 140 mm in height, while the number of polyps is reduced tenfold. Most often, only three polyps are formed on the trunk of these codonia, the length of which when extended reaches 22 mm. Finally, an expedition on the Vityaz vessel in many places in the Pacific Ocean and in the Bering Sea at depths from 2843 to 4070 m found numerous colonies of a hitherto unknown species K. biflorum, on the trunk of which there were only two very large polyps. The height of these colonies reached 150 mm, and the length of the polyps in the expanded state was 33 mm. Two years ago, during the Vityaz’s voyage in the Indian Ocean, we managed to raise two very interesting colonies from a depth of 3500 and 4800 m umbellul, which also turned out to be representatives of a new species. At the top of the trunk of these umbellulae there was only one polyp, but of enormous size. When unfolded, its length reaches 125 mm. Close species of umbellula - U. thomsoni and U. dutissima, living at depths of 1300-5600 and 1000-3000 m, have 4-7 and 3-14 polyps of much smaller sizes, respectively. Thus, a new species of umbellula, named U. monocephalus (single-headed), represents an extreme example of this series. The process of reducing the number of polyps while simultaneously increasing their size can also be observed in representatives of the same species living at different depths of the ocean. The difference in the structure of the colonies, the number and size of polyps of individual forms living in shallow water and at great depths of the ocean can be so great that such forms, which are very different from each other, were considered by many scientists as independent species.
So far we have spoken of colonies from the point of view of their general structural plan. Methods for strengthening colonies and their branching and arrangement were considered. Now we need to dwell on another very important property of coelenterate colonies. In these peculiar animals, polyps do not always have the same structure. Very often, individual polyps have different shapes and play different roles in the life of the colony. Colonies on which polyps of different structure and functions are formed are called polymorphic.
Most often, polymorphism is observed among hydroids. Due to the alternation of generations, or metagenesis, which is very characteristic of these coelenterates, in the colonies, in addition to the feeding polyps (which were discussed above), individuals of the medusoid generation also bud off. The polyps that are adapted for budding of jellyfish differ significantly from the usual colony-feeding polyps. They lack tentacles and mouth openings. But polymorphism can go much further. Then extremely highly modified polyps are formed on the coenosarcium, which have a protective function. They have the form of simple or branched threads, also without a mouth or tentacles. But their stinging capsules acquire special development, with the help of which the polyps can strike an enemy or prey. These polyps, called dactylozoids, are arranged in a specific pattern around the feeding polyps and the medusa-producing polyps.
Polymorphism is also characteristic of many eight-rayed corals. But here it has a limited scope, since the function of sexual reproduction passes to ordinary nursing polyps, or autozoids, and the ameduzoid generation is completely absent. With regard to coral polyps, it is more correct to talk not about their polymorphism, but about dimorphism, since in most of their colonies only two types of polyps are formed: the autozooids mentioned above and very small polyps, often completely devoid of tentacles, called siphonozoids. In these zooids, the partitions in the intestinal cavity, so characteristic of coral polyps, are significantly reduced, but the siphonoglyph is very developed. Siphonozoids cannot obtain food and digest it. The function of siphonozoids is that, with the help of the beating of the cilia of siphonoglyphs, they create a powerful current of water in a system of channels connecting the individual parts of the colony. With this flow of water, nutrients and oxygen necessary for respiration are carried throughout the colony. Thus, we see that in eight-rayed corals the polymorphism of polyps is rather weakly expressed. In colonial six-rayed corals, polymorphism is not observed at all.
The highest type of polymorphic colonies are not hydroids and coral polyps, but a very peculiar group of free-swimming coelenterates - siphonophores. These colonial animals, which are extremely diverse in their structure, color and appearance, consist of a hollow trunk on which numerous polypoid and medusoid individuals bud, performing various functions and differing from each other in their shape and structure.
At the very top of the colony, a medusoid individual buds - a pneumatophore, or swim bladder. It can be very small (no more than 1 - 20 mm), but sometimes reaches 20-30 cm. In some siphonophores, the pneumatophore is closed, in others it has a pore. The shape of the pneumatophore varies from almost spherical, oval or pear-shaped to asymmetrical. Sometimes various outgrowths, or ridges, form on the surface of the pneumatophore. The pneumatophore serves as a hydrostatic apparatus for most siphonophore colonies. The animal can regulate the amount of gas contained in the pneumatophore, releasing it through a pore equipped with an adductor muscle, or again producing it through special glandular cells. By increasing or decreasing the volume of the pneumatophore, the siphonophore is able to rise to the water surface or sink into the depths of the ocean. For some siphonophores that live on the surface of the sea, the pneumatophore serves as a sail.
Immediately under the pneumatophore of most siphonophores, other medusoid individuals bud - nectophores, or swimming bells (from 10 to 400 in each colony). In some species there is no pneumatophore, and then the nectophores are located in the uppermost part of the colony. Nectophores, successively contracting and expanding, move the colony in a horizontal direction, but can, like pneumatophores, contribute to the movement of the siphonophore up or down.
The remaining individuals of the colony are located in the lower half of the trunk. Among them, one can distinguish feeding polyps, or gastrozoids, at the base of each of which there is only one long tentacle, called a lasso. The lasso, as a rule, branches, and on its branches there are clusters of stinging cells. If there are no branches, then the stinging cells are located in the form of plaques on the lasso itself.
In addition to gastrozoids, many siphonophores have polyps of another type - palpons and cystozoids. They are simpler than gastrozoids and sometimes even lack a mouth. Their tentacles do not branch, but, like lassoes, are equipped with stinging cells. It is assumed that the palpons and cystozoids perform sensory and excretory functions.
Most siphonophore colonies have medusoid individuals that play a protective role. These are the covering plates. They are very much modified and do not look like jellyfish at all.
Finally, a permanent component of the colony are individuals that perform the function of sexual reproduction. They can have the appearance of well-developed jellyfish with radical canals and an oral proboscis, in the walls of which reproductive products are formed. In some siphonophores, such jellyfish can even break away and swim freely until the reproductive products in them mature. But much more often, the umbrella of jellyfish is reduced and even merges with the oral proboscis.
It is very important to note that in the most complex siphonophores, some of the listed individuals are located on the trunk in small groups called cormidia. Each cormidium necessarily includes polyps that perform nutritional functions and reproductive jellyfish. Cormidia can separate from the main colony and lead an independent life. In this case, cormidia are called eudoxia or ersei.
Previously, individual cormidia were even considered independent species of siphonophores. Now, after the structure of various coelenterate colonies has been examined, it is interesting to return again to the question of the subordination of individual parts to the individuality of the entire colony, which was already touched upon at the beginning of this section.
In the process of gradual complication of the structure of the colonies, individual polyps lose more and more of their individual significance and only perform the role that is dictated by the entire colony as a whole. This subordination of individual polyps is especially clearly manifested in polymorphic colonies. Polyps in such colonies are no longer potentially capable of maintaining an independent lifestyle. In polymorphic colonies, the functions of its members are strictly distributed among themselves and at the same time closely related. Gradual integration of colonies is observed in all major groups of coelenterates. In the class of hydroids, the most perfect colonies in this regard are siphonophores. In fact, their polymorphic colony has turned into a real independent organism, capable of active directed movement. Among coral polyps, the highest degree of subordination of individual members of the entire colony as a whole is possessed by sea feathers, which also turn into an independent, perfectly harmonious organism. And sea feathers are extremely characterized by the ability to independently, actively move along the ground, and the ability to gain a foothold in it. The concept of an individual is already quite applicable to siphonophores and sea feathers, since these colonies represent a single morphophysiological whole, in which the individuality of individual parts is completely absent.
How is the general regulation of colony activity carried out in coelenterates, these most simply structured animals? This question is still waiting to be resolved, since at present the knowledge in the field of anatomy and physiology of coelenterates is still insufficient for this. But many experiments and observations indicate that a general regulatory mechanism exists in colonies and turns out to be more complex, the greater the degree of integration of the colony. In the simplest cases, for example, in colonies of hydroids, in which the nervous system is at a very low stage of development, regulatory activity is barely expressed. This is apparently explained by the fact that individual polyps of the colonies are interconnected by coenosarcal canals. In any case, there is still no information about the presence of a general colonial nervous system in hydroids; irritation from one of the polyps of the colony is not transmitted to the rest. In the most simply organized colonies of coral polyps, the general colonial nervous system is also absent and the transmission of irritations also occurs only through the coenosarcal canals. But due to the peculiarities of the conduction system, the transmission of irritation occurs at a level higher than that of hydroids. For example, irritation of one of the polyps of the Alcyonium colony is already transmitted to neighboring polyps. True, the wave of contraction of polyps spreads surprisingly slowly and, as a rule, only affects branches located in the immediate vicinity of the polyp to which the irritation was applied. But as the structure of colonies and their integration become more complex, the nervous system of coelenterates also becomes more complex. In polymorphic colonies of the highest type, a general colonial nervous system is already formed, connecting the most distant parts of the colony. A nervous system permeating the entire body of the colony is observed, as one would expect, in siphonophores among hydroid polyps and in sea feathers among corals. In these coelenterates, irritation of one polyp causes a sharp reaction of the entire colony. As a rule, this reaction is expressed in the reduction of the colony. In siphonophores, the tentacles quickly retract and the pneumatophore or swimming bells contract. The body begins to dive or changes the direction of its movement, trying to get away from danger. Irritation of one of the many polyps sea pen within a few seconds it spreads throughout the colony. With several strong contractile movements of the basal expansion, the colony is pulled deep into the ground. The entire upper part of the colony, remaining above the ground, is also reduced. The polyps quickly disappear into the thickness of the trunk, while waves of phosphorescent light run throughout the colony.
Cohabitation of Coelenterates with Other Organisms
Explaining the emergence of various forms of cohabitation is not so difficult. In organisms such as coelenterates, which lead an attached existence and have a branched tree-like form, fouling of some organisms by others easily occurs. In the sea you can always find a strong tree-like colony rising high above the ground, the branches of which are covered with various colonies of hydroids, alcyonaria, bryozoans or single organisms - sea anemones, ascidians, brachiopods. Coelenterates, both colonial and solitary, often overgrow the rough rough surface of ascidians and their long legs, attach to the surface and to individual long spicules of sponges, cover algae thalli and leaves of sea grasses, etc. But most of them prefer some kind of one specific species, which they use as a substrate. For example, colonies of the hydroid Filellum serpens can be found on the trunks of almost all other hydroids, but usually this creeping form develops on several types of sea spruce - sertularium. Another hydroid, Obelia Nexuosa, usually lives on the thalli of the brown algae Fucus, although colonies obelia can easily be found on other algae and on the leaves of the sea grass Zostera. Apparently, in this way the transition from non-selective fouling to permanent forms of coexistence (sinoikia) occurs in nature.
The hydroid Perigonimus is a very characteristic cohabitant of some bivalves. Usually this hydroid settles on the edge of the mollusk shell that covers its siphons. The mollusks that serve as hosts for Perigonimus are, by the type of their diet, filter-feeding organisms that feed on tiny organic particles suspended in seawater. In order to provide itself with food, the mollusk must continuously drive water through its mantle cavity, entering through the inlet and leaving through the outlet siphon. The water currents created in this process obviously make it easier for the hydroid to catch food. Another creeping hydroid, Hydractinia echinata, is a cohabitant of gastropods, most often the predatory mollusk Natica. The hydroid Ptilocedium repens, living in the Tasman Sea, has adapted to exist on sea pen Sarcophyllum. This is a very curious case of commensalism, for sea feathers very rarely become fouled.
Sinoikia is also present among small eight-rayed corals - alcyonarian. For example, Parerythropodium coralloides, which lives in the Adriatic Sea, lives exclusively on the branches of living colonies gorgonians Eunicella verrucosa. In the English Channel, the same gorgonian again serves as a host, but now to a different commensal - sea anemones Amphianthus dohrnii.
Among the gorgonians themselves, there is apparently no real synoicia, and they can only be hosts for other marine organisms. For example, two other species of gorgonians - Primnoa resedaeformis and Paragordia arborea, widespread in the temperate zone of the northern hemisphere, in the Norwegian Sea almost always serve as hosts for colonies of Epizoanthus norvegicus. Yellow Primnoa is also the host of the yellow-colored form of the sea anemone Ptichodactys patula. A special form of this sea anemone, which is blue in color, settles on the blue gorgonian - Paramuricea placomus.
There are a lot of synoicia among the zoantharians of colonial six-rayed corals, which never form their own skeleton, but the outer surface of which is often encrusted with all sorts of foreign particles, both organic (shells of foraminifera and diatoms, spicules of sponges and coral polyps) and inorganic origin (grains of sand, small fragments calcareous rocks, etc.). Palythoa, one of the species of these coelenterates, is a cohabitant of the gorgonian Corallium inutile that lives in Japanese waters. The host coral serves for Palythoa not only as a substrate for attachment and separation from the ground, but, what is much more curious, also as a supplier of material for the construction of the external skeleton: Palythoa encrustes the surface of its colony with calcareous gorgonian sclerites. Another species of zoantharium common in the Adriatic Sea is Parazoanthus axinellae - a cohabitant of two species of sponges from the genus Axinella, although, as an exception, it also settles on some other sponges.
Synoikia is also widespread among sea anemones. It was already reported above that the yellow-colored and blue forms of the sea anemone Ptychodactis are, respectively, cohabitants of the gorgonians Primnoa and Paramuricea. There are many examples of this kind that can be given. Therefore, we will focus on the most interesting ones. During deep-sea trawling conducted from the Soviet research vessel Vityaz in the northwestern part of the Pacific Ocean at a depth of 3-4 thousand m, the spadefoot mollusks Scaphopoda, whose conical saber-shaped shells surprisingly resemble the shape of a walrus tusk, were very often encountered. Colorless thin-walled sea anemones with a small number of tentacles were found on almost all the shells of the collected mollusks. These sea anemones settle at the level of the lower third of the shell, that is, slightly above the part of the shell that is constantly submerged in the ground. It can be assumed that when a mollusk moves along the ground, the smallest organisms living in the upper layer of soil emerge, disturbed, and become victims of the sea anemone. The cohabitation of this anemone with a spadefoot mollusk is so common that the occasional discovery of shells free of anemones caused surprise every time. These animals apparently never settle on other substrates.
Even more interesting cases of synoicia among deep-sea anemones are also known. At the beginning of the 20th century. As a result of deep-sea trawling in the Atlantic Ocean conducted by the Oceanographic Institute of Monaco, two specimens of the holothurian Pseudostichopus villosus were caught. It is not difficult to imagine the surprise of scientists when they discovered two small dense sea anemones rooted there on the lower surface of these holothurians! However, the cohabitation of the sea anemone, named Sicyopus commensalis, is apparently not accidental. On both specimens of holothurians, sea anemones settled in the same place - in front and slightly to the side of the mouth opening surrounded by short tentacles. These flattened holothurians continuously crawl on the surface of the ground, collecting particles of organic detritus with their tentacles. In this case, Sicyopus commensalis finds itself in the position of the vanguard, the first to develop new “hunting grounds.” It is curious that under the sea anemone removed from the body of the holothurian, a small hole was discovered, into which the sea anemone was, as it were, pressed. In this regard, it is interesting to note observations made on a number of other sea anemones, which showed that their sole secretes a special secretion that easily dissolves the organic substrate on which they settle. For example, the sea anemone Cereus pedunculatus, which has attached itself to the shell of a mollusk, gradually dissolves it directly under its sole and after some time falls into the shell through the hole formed in it. It is possible that the pit in the body of the holothurian, in which the sea anemone is strengthened, has the same origin. In this case, the roommate does not really spare his owner. But the anemones that settle on the deep-sea sea pen Kophobelemnon biflorum treat their host even more ruthlessly. These sea feathers were found for the first time and in large quantities by the expedition ship Vityaz. According to available data, they live along the coast of the North Pacific Ocean at a depth of 3-4 thousand de, preferring the silted slopes of deep-sea depressions. One or more sea anemones closely related to Sicyopus were found on each of the collected specimens of K. biflorum. Just as in the case of cohabitation with holothuria, these sea anemones destroy the soft tissues of the sea feather with their soles, penetrating deeper and deeper into its body. Finally, their base reaches the calcareous axial core of the colony and tightly envelops it. Commensalism of this type turns out to be destructive for the sea pen. All his soft tissues die and fall off. And then the winning commensal finds itself sitting on the smooth axial rod of the dead colony at a height of 7-10 cm above ground level. In the collection of K. biflorum that we reviewed, collected by the Vityaz expedition, one can find all stages of the described process. There are magnificent specimens of feathers on which young sea anemones have just settled, and there are also colonies that are already in decline. On two specimens of K. biflorum, only the uppermost part with two large autozoa was preserved from its soft tissues, and the lower part was completely destroyed by sea anemones, which had already become very large.
Finally, the collection also contains several cores of dead colonies, tightly covered by the soles of former cohabitants.
Just like synoikia, symbiosis arose from the simple fouling of some organisms by others. In the case of mutual benefit of such cohabitation, an instinct or certain adaptations in the structure could be developed in the future, facilitating the meeting of symbiont organisms. Finally, symbiosis becomes a necessity for such organisms: the existence of each of them is so closely dependent on the existence of the other that their life separately turns out to be difficult or impossible.
This can be illustrated by a number of examples. Thus, the hydroid Hydractinia echinata, as mentioned above, usually grows over the shells of predatory gastropods, being their commensal. But often N. echinata also grows over shells that serve as homes for hermit crabs. The hydrorhiza hydroid, which grows over time, gradually turns into a continuous plate, and its edge at the same time outgrows the mouth of the shell and hangs over the body of the hermit crab. Such cohabitation becomes beneficial for both the hydroid and the hermit crab. The constant movement of the crayfish along the bottom of the reservoir in search of food contributes to better gas exchange of the hydroid, and the disturbed remains of food devoured by the crayfish are easily captured and eaten by the polyps of hydra anemones. At the same time, the growth of the hydroid disguises the shell of the hermit crab, which is certainly useful for it. In addition, the crayfish does not have to change its home often, because as the crayfish grows, the hydrorhizal plate also grows, serving the hermit crab as quite reliable protection. It is easy to see that the cohabitation described now is the simplest case of symbiosis. There is no active stakeholder here: the hydroid accidentally settles on a shell house occupied by a hermit crab. The latter is not looking for a shell that is already overgrown with a colony of hydractinia. Each of the partners, both cancer and hydroid, can exist completely independently.
Classic examples of the symbiosis of two organisms are provided by the cohabitation of hermit crabs and sea anemones. But even in these cases, three stages of gradual complications in relations between partners can be distinguished. In the simplest version, the hermit crab is not yet able to remove an anemone that has settled on some substrate and transfer it to its house. He only abandons his old house if he accidentally finds a suitable empty shell with an anemone already settled on it. This is how, for example, the hermit crab Eupagurus excavatus behaves if it accidentally comes across an empty shell suitable for it, on which the sea anemone Calliactis parasitica has settled. A crayfish does exactly the same thing when it finds an empty shell with Hormathia corenata or even Actinia equina sitting on it, although both of these sea anemones lead a completely independent lifestyle and do not enter into symbiosis with other animals.
The life together of an anemone and a hermit crab provides undoubted advantages to each of the partners. Now the sea anemone no longer needs to be in constant and tense anticipation of catching food. She easily catches with her tentacles the agitated remains of the hermit crab's food, in search of which it continuously moves along the bottom of the reservoir. The cancer is now under the reliable protection of the stinging capsules of the sea anemone - a formidable weapon for most enemies of hermit crabs.
A higher level in the development of symbiotic relationships is shown by the cohabitation of the same Calliactis parasitica with another hermit crab - Pagurus arrosor. A hermit crab should not look for an empty shell with an anemone already sitting on it. He is able to remove it from any substrate, transfer it to his house and strengthen it on it. When a hermit crab’s old house turns out to be too small and he is forced to change it to a new one, he removes the sea anemone from the old one and transfers it to his new house. This happens as follows. With its walking legs of the first and third pairs, the hermit crab begins to stroke and pat the sea anemone. It would seem that the sea anemone, which responds to any irritation by contracting its body and throwing out stinging threads, should act in the same way when the crayfish begins to pat it. In this case, the sea anemone behaves differently. At the first touch of the crayfish, however, it begins to shrink, but then it opens again and does not throw out stinging threads. Even a fully contracted sea anemone begins to bloom if a hermit crab begins to stroke it. After the sea anemone has completely blossomed, its future companion begins to stroke the sole of the sea anemone. With this stroking, the cancer achieves contraction of the sole and its separation from the substrate. Then the crayfish can only place its shell closer to the sole of the sea anemone and roll the sea anemone onto it.

However, there are indications that after the separation of the sole has occurred, the sea anemone itself bends towards the hermit shell and covers it with its tentacles. Then it turns over again and is attached to the shell with its sole. In those cases when a hermit crab finds a sea anemone that is not completely firmly entrenched, he does not even have to pat and stroke it - the described process of transplanting the sea anemone proceeds without his help.
The sea anemone secretes a mucous membrane at its sole, which helps to more securely strengthen the sea anemone on the house of the hermit crab. However, this thin membrane cannot grow beyond the edge of the shell and thereby increase its volume. After some time, the hermit has to change his old house, which has become cramped for him, to a new, more spacious one, and again move his companion into it. If there is enough space left on the shell, an enterprising crayfish may acquire another sea anemone. Cases are described when Pagurus arrosor carried up to 8 actils on its shell.
The best example of the highest type of symbiosis between sea anemone and hermit crab is the cohabitation of Adamsia palliata with Eupagurus prideaxi. Adamsia, distributed in the Mediterranean Sea and in the temperate waters of the eastern Atlantic Ocean, leads an independent existence only at the very beginning of its life. Young adamsia have a cylindrical body, typical for sea anemones, with a rather wide sole, with which they attach to stones or empty shells of mollusks. Very rarely do they manage to grow to 1 cm in height: as a rule, even before that they are captured by hermit crabs. It has not yet been fully established whether this sea anemone is capable of leading an independent lifestyle even in adulthood, unless by some chance it enters into symbiosis with a hermit. However, it is very difficult to imagine a solitary Adamsia, knowing the habits of the hermit E. prideaxi. Among his relatives he is the most active and pugnacious. That's why he prefers the smallest shells as his home. The shells of the predatory gastropods Nassa and Natica, which he usually uses, barely cover his soft abdomen. In an adult, large crayfish, such a house protects only the most posterior part of the abdomen. As for the cephalothorax of cancer, it is never covered by a shell. Eupagurus can protect itself from enemies and predators in only one way - to find and enter into symbiosis with Adamsia. Young cancer is in constant search of adamsia. In searching for sea anemones, it is not so much his vision that helps him, but his sense of touch and the ability to detect certain chemical irritations emanating from the sea anemone. In an aquarium, this hermit crab detects young Adamsia even if it is covered with a cloth cap. As soon as he spots a single young sea anemone, he quickly rushes towards it. At this time, it seems that nothing can stop him. Approaching the adamsia, Eupagurus grabs it tightly with its claws and does not let go for 10 minutes. It would seem that such treatment should make Adamsia shrink. In reality this does not happen. On the contrary, the sole of the young adamsia begins to separate from the substrate. After this, the crayfish, without unclenching its claws, transfers it to its house.
When a young hermit crab, who has not yet had time to acquire a companion, meets his more successful relative, he immediately enters into battle with him for the possession of adamsia. If the defender turns out to be weaker, he throws the shell and takes off running, leaving the aggressor both his home and his sea anemone.
The hermit crab E. prideaxi strengthens the adamsia on the shell so that its oral disc is directed forward, and its entire body is behind and just below the oral apparatus of the crayfish itself. In this position, the sea anemone can easily catch and eat the remains of food consumed by the cancer. At the same time, this position of the sea anemone causes it to change its body shape over time. The fact is that its growth cannot proceed evenly in all directions. The spread of the sole along the shell further down and back is prevented by the constant friction of the shell against the ground. The sea anemone cannot grow forward; the moving legs of the crayfish interfere with this. The sea anemone grows sideways and upward, along the edge of the mouth of the shell. Her body therefore takes on a crescent shape. If the hermit crab has not acquired a second sea anemone, then the lateral lobes of the sole, rising higher and higher along the shell, can close, ringing the mouth of the shell. If there are two sea anemones on the shell, then the edges of their soles also meet each other on the upper and lower surfaces of the shell. Adamspi has another property that is extremely useful for hermit crabs: the sole of Adamsia, like the sole of Kalyaktis, secretes a dense mucous plate, which, however, in Adamsia quickly hardens and takes on a horny structure. As the sea anemone grows, this plate outgrows the edge of the shell mouth and hangs over the body of the crayfish. The volume of the house thereby increases all the time, and the fast-growing crayfish does not have to change its house. Large adult adamsias reach a width of 5-7 cm. The house of the hermit crab has a much more modest size. It turns out that Adamsia ultimately sits not so much on the surface of the cancer’s original shelter, but on the horny ring around its body, which it itself built.
Such a house is also very convenient for a hermit crab, because it is disproportionately lighter than a thick-walled mollusk shell and is quite durable. At the same time, it is elastic and does not impede the crayfish’s movements at all.
After strengthening the adamsia on the shell, not only the shape of its body changes, but also its color. Young adamsias are bright pink-red. But in adult adamsias that have entered into symbiosis with pagurus, the color of the body changes and becomes yellow-pink, of different shades, but the same as the color of the cephalothorax and the upper segments of the walking legs of the crayfish. The dorsal lobes of the sea anemone's sole are the most brightly colored; closer to the sides they become lighter. The oral disc and tentacles, invisible from above, are almost completely colorless (see color table 9).

The mutual benefit of the symbiosis of Adamsia palliata and Eupagurus prideaxi is obvious. A hermit crab, almost the entire body of which is not covered by a shell, would have long ago become a victim of some predator if it were not reliably protected by an anemone. Even such ferocious predators as cephalopods do not dare to attack the hermit, fearing the adamsia's terrible weapon - its stinging threads. Adamsia, in turn, does not care about food - this is the direct responsibility of its owner. The constant movement of the crayfish along the ground is also very beneficial for the sea anemone, as it improves the conditions for gas exchange. It is not necessary, however, for the sea anemone to attach to the shell or shells of crustaceans. In nature, other forms of symbiosis between sea anemones and crayfish are developed. Sea anemone can then be used not only as a weapon of defense, but also as a tool for obtaining food. One of the Mediterranean crabs is known for the fact that it constantly holds two sea anemones in its claws, which serve it both as protection from enemies and as a tool for obtaining food. With its tentacles armed with stinging capsules, the sea anemone grabs passing prey. But her crab owner shamelessly takes part of the prey for himself. The only advantage of an anemone leading such a lifestyle is that it constantly moves from one place to another. The symbiosis in this case is somewhat one-sided.

In all the communities described so far, the active side was crustaceans and the passive side was sea anemones. But it also happens the other way around. For example, the sea anemone Autholoba reticulata, which lives on the west coast of South America, itself strives to enter into symbiosis with crustaceans, preferring the Hepatus crab to others. This sea anemone, like its other relatives, first settles on stones, attaching itself to them with its sole. But then it turns over with its oral disc down and holds on to the substrate with its tentacles. Its sole comes off the substrate. In such an unusual position for an anemone, she awaits the approach of her future companion. After a while, her patience may be rewarded, and then her sole firmly grasps the leg of a crab crawling past. Then the sea anemone crawls onto his shell.
There is an equally interesting symbiosis between sea anemones and fish. The shallow waters of the western coast of Australia are home to the largest anemones in the world, Stoichactis, whose oral disc reaches a diameter of 1.5 m. On the surface of the disc, countless tentacles of these anemones are located in several concentric rows. Among the tentacles or in close proximity to them are a couple of very brightly colored amphiprion fish. According to the observations of a number of authors, it is impossible to find a single sea anemone near which these fish would not stay, just as it is impossible to find fish far from the sea anemones. Even in search of prey, fish never move more than a meter away from sea anemones. At the slightest danger, they hide in the forest of long tentacles of sea anemones. If you put predatory fish in an aquarium with sea anemones and amphiprions, they can live together for a very long time, but as soon as the sea anemone is removed, the amphiprions become victims of predatory fish. Amphiprions cannot swim quickly or find protection in algae, and their very bright coloring and beautiful pattern of white and black stripes on a red or golden background are too noticeable for the enemy.
Under natural conditions, this same bright coloring benefits the fish, attracting prey to them. Since the fish grab prey in the immediate vicinity of the tentacles and mouth opening of the sea anemone, eat it here and, naturally, lose its remains, they thereby supply food to the sea anemone. In addition, staying among the tentacles, the fish, by constantly beating their fins, create currents of water that improve the conditions for gas exchange of the sea anemone.
In addition to these fish, among the tentacles of sea anemones there are often several species of shrimp and a small crab that imitates fish in color.
The symbiosis of coelenterates with unicellular algae zooxanthellae will be discussed in the chapter on coral structures.



At first, the polyps have no mouth and feed on yolk reserves, but on the 4-5th day of independent life they develop a mouth and the polyps, with the help of tentacles lined with stinging cells, begin to catch oligochaete worms, rotifers and other small freshwater animals. The four short tentacles of the polyp are used for attachment to the bottom and for movement; the polyp uses longer tentacles to catch prey.
Throughout the summer, polyps reproduce by division. Before division, the number of tentacles in the polyp doubles, and then a longitudinal constriction appears on its body, and soon it is divided in two.
THE IMPORTANCE OF CENTEROCAVITIES IN PALEONTOLOGY
Among the most ancient coelenterates, organisms lacking a well-developed skeleton predominated. There are known finds of several scyphoid jellyfish from Cambrian and Cretaceous deposits.
Only the remains of extinct reef-building corals, which had a massive calcareous skeleton, are often found in a wide variety of geological deposits. More than 5,000 species of fossil corals have been described. They were found in the form of separate imprints, in the form of large fossil reefs. The most ancient corals were archaeocyathids from the Lower Cambrian and heliotides from Silurian deposits.
Beginning with the Silurian period, a group of very peculiar four-rayed corals(Tetracorallia). These were solitary or colonial corals of a conical shape, with a widened upper cup. Four primary partitions are clearly visible on its surface. The partitions (or septa) divided the calyx into four sectors, each of which in turn was divided by secondary partitions.
True madrepore reef-building corals appeared only in the Cretaceous period, and modern families of these corals appeared only in the Quaternary period.
Despite the numerous and varied finds of fossil corals, the group of coelenterates as a whole constituted a very small part of the accumulated paleontological material and therefore could not play any significant role in paleontological reconstructions. However, recent research conducted in the Ediacara Valley in South Australia has forced scientists to change this opinion about the importance of coelenterates in the fossil record.
Until quite recently, no one knew what the ancestors of those invertebrate animals that reached significant development at the beginning of the Cambrian period were.
And so, during excavations of Precambrian rocks in the Ediacara Valley, it was finally possible to collect more than 6,000 samples of fossil invertebrates that inhabited the seas in the Proterozoic era. Among the specimens collected were prints of jellyfish from six different genera, soft corals and coral polyps that resembled modern sea feathers, and a variety of other animals. Among the numerous coral prints, there was not one that would indicate the presence of a massive calcareous skeleton. The tissues of the most ancient coral polyps were supported only by simple calcareous bodies - spicules.
All these finds still require close study. However, it can already be said that the assumption that Precambrian animals were devoid of a skeleton or were not covered with a shell and therefore remained unknown until now, apparently turned out to be fair. In addition, the study of the collected remains suggests that the absence of shells or internal skeletons in Precambrian animals is explained not by any special living conditions at that time hundreds of millions of years removed from us, and not by any sharp difference in the way of life of these animals. The absence of a skeleton, apparently, should be explained by the fact that the oldest invertebrate animals had not yet developed the mechanism of calcium metabolism, as a result of which the organisms were subsequently capable of forming a massive calcareous skeleton, shells and shells.
Now, thanks to the works of zoologists of the Russian school - I.I. Mechnikov, V.N. Beklemishev and A.A. Zakhvatkin - the earliest periods in the evolution of coelenterates have become clear. It can be considered established that the ancestors of modern coelenterates were primitive multicellular animals that resembled planulae of modern species.
Unfortunately, among the paleontological finds there are no remains of these tiny, skeletonless metazoan ancestors, and therefore it is possible to imagine a picture of the early evolution of coelenterates only by drawing analogies and studying individual development in detail.
We have the right to assume that the next stage in the evolution of coelenterates after the planula was the polyp.
Many sessile organisms are characterized by the ability to reproduce vegetatively and form colonies. This feature allows them to better fight for existence: polyps jointly catch and assimilate prey, and jointly defend themselves from enemies.
At the early stages of development of the coelenterates, they were divided into two large branches. Polyps appeared, the gastric cavity of which was divided into chambers by partitions. This contributed to more intensive digestion. Subsequently, coral and scyphoid polyps evolved from these polyps. The same polyps that did not have partitions in the intestinal cavity are hydroid. However, the attached lifestyle also had its drawbacks: the offspring of sessile animals always settled next to their parents. This created overpopulation and prevented animals from conquering new living spaces.
The appearance of mobile sexual individuals jellyfish was a very significant step towards the evolution of coelenterates; this feature, along with the development of the stinging apparatus, is one of the main reasons for the prosperity of the type.
This very ancient group of organisms is undoubtedly at the same time one of the most prosperous. Of course, one cannot expect that in the future any highly organized animals will arise from the coelenterates, but their ability to adapt to diverse living conditions will allow them to continue to successfully compete with much more highly organized inhabitants of the sea.
- radial (Coelenterata, Radialia), section of invertebrate animals of the supersection of eumetazoans. 2 types: cnidarians and ctenophores. Sometimes K. called. only cnidarians or combine both types into type K. .(General characteristics, variety of types
The type of coelenterates has about 9 thousand species. They originated from colonial protozoa - flagellates and are distributed in all seas and freshwater bodies. The type of coelenterates is divided into three classes: hydroid, scyphoid and coral polyps.
The main aromorphoses that contributed to the appearance of coelenterates:
- the emergence of multicellularity as a result of specialization and association of interacting cells;
- the appearance of a two-layer structure;
- the occurrence of cavity digestion;
- the appearance of body parts differentiated by function;
- the appearance of radial symmetry.
Coelenterates lead an aquatic, free-living or sedentary lifestyle. These are two-layer animals, in ontogenesis they form two germ layers - ecto- and endoderm, between which there is mesoglea - the supporting plate. Their internal cavity is called the gastric cavity. Here food is digested, the remains of which are removed through the mouth, surrounded by tentacles (in hydras).
Hydroid class
A representative of this class is the freshwater hydra.
Hydra is a polyp about 1 cm in size. It lives in freshwater bodies, attaching itself to the substrate with its sole. The front end of the animal's body forms a mouth surrounded by tentacles. The body of the hydra is covered with ectoderm, consisting of several types of cells:
- epithelial-muscular;
- intermediate;
- stinging;
- sexual;
- nervous.
Hydra endoderm consists of epithelial-muscular, digestive cells and glandular cells.
Left - Diagram of the location of nerve cells in the body of the hydra. (according to Hesse). On the right - Stinging cells: A - in a resting state, B - with the stinging thread thrown out (according to Kuhn): 1 - nucleus; 2 - stinging capsule; 3 - cnidocil; 4 - stinging thread with spines; 5 - spikes
Important features of coelenterates:
- the presence of stinging cells in the outer layer. They develop from intermediate ones and consist of a stinging capsule filled with liquid and a stinging thread placed in the capsule. Stinging cells serve as weapons of attack and defense;
- cavity digestion with preservation of intracellular digestion.
Hydras are predators that feed on small crustaceans and fish fry.
Breathing and excretion are carried out over the entire surface of their body.
Irritability manifests itself in the form of motor reflexes. The tentacles react most clearly to irritation, since nerve and epithelial-muscle cells are densely concentrated in them.
Hydras reproduce by budding and sexually. The sexual process occurs in the fall. Some intermediate cells of the ectoderm turn into germ cells. Fertilization occurs in water. In the spring, new hydras appear. Among the coelenterates there are hermaphrodites and dioecious animals.
Many coelenterates are characterized by alternating generations. For example, jellyfish are formed from polyps, larvae - planulae - develop from fertilized jellyfish eggs, and polyps develop from the larvae again.
Hydras are able to restore lost body parts due to the reproduction and differentiation of nonspecific cells. This phenomenon is called regeneration.
Class Scyphoid
This class unites large jellyfish (representatives - cornerot, aurelia, cyanea).
Jellyfish live in the seas. In their life cycle, sexual and asexual generations naturally alternate. The body is shaped like an umbrella and consists mainly of gelatinous mesoglea, covered on the outside with one layer of ectoderm, and on the inside with a layer of endoderm. Along the edges of the umbrella there are tentacles surrounding the mouth, located on the underside. The mouth leads into the gastric cavity, from which radial canals extend, which are connected to each other by a ring canal. As a result, the gastric system is formed.
The nervous system of jellyfish is more complex than that of hydras.

Rice. 34. Development of scyphomedusa: 1 - egg; 2 - planula; 3 - single polyp; 4 - budding polyp; 5 - dividing polyp; 6 - young jellyfish; 7 - adult jellyfish
In addition to the general network of nerve cells, along the edge of the umbrella there are clusters of nerve ganglia, forming a continuous nerve ring and special balance organs - statocysts. Some jellyfish develop light-sensitive eyes, sensory and pigment cells corresponding to the retina of higher animals.
Jellyfish are dioecious. Their gonads are located under the radial canals or on the oral stalk. Reproductive products exit through the mouth into the sea. From the zygote, a free-living larva develops - a planula, which in the spring turns into a small polyp.
Class Coral polyps
Includes solitary (anemone) or colonial forms (red coral). They have a calcareous or silicon skeleton formed by needle-shaped crystals, live in tropical seas, reproduce asexually and sexually (there is no jellyfish stage of development). Clusters of coral polyps form coral reefs.
 Photo gallery —> “Butterflies”
Photo gallery —> “Butterflies”