Topic: “Chemical composition of the cell. Basic biopolymer molecules of living matter." Grade 11. Biology teachers of the first category: Kovalenko V.V. Municipal Educational Institution Secondary School 149 Topic: “Chemical composition of the cell. Basic biopolymer molecules of living matter." Grade 11. Biology teachers of the 1st category: Kovalenko V. V. Municipal Educational Institution Secondary School 149
Objectives: consolidate knowledge: on the basic properties of the molecular level; according to the characteristics of the chemical composition of living cells; about the structural features of biological molecules and their functions in living cells; about the need for adequate nutrition to replenish the body and its cells with all the necessary substances.

Differences between living and non-living nature Movement speed up to 70 km/hour Speed 60 km/hour Energy due to the breakdown of organic substances. Consumes oxygen Emits carbon dioxide Basic chemical elements: carbon, oxygen, nitrogen, hydrogen Basic chemical elements: iron, aluminum, copper, carbon Cheetah Subcompact car

Answer the questions What is the significance of the molecular level of living matter? Briefly describe the physicochemical and biological features of biological molecules? What are the basic processes at the molecular level of life? So what are the differences in the chemical composition of living cells? Elementary? Molecular?


The study of the elemental composition of a cell confirms the unity of living and nonliving nature. Living organisms contain the same chemical elements that make up inanimate bodies. From 70 to 90 of the 107 (110) elements that make up the periodic system of D.I. were found in cells. Mendeleev. Approximately 40 elements take part in metabolic processes and have pronounced biological activity. These elements are called biogenic. Biogenic elements are chemical elements that, when included in cells, perform biological functions.

Most of the inorganic substances are found in the cell in the form of salts - sulfuric, hydrochloric, phosphoric and other acids. Mineral salts play an important role in the development of living organisms. Their deficiency or excess can lead to the death of the body. Salts can be present in the cell either in the form of ions or in a solid state. Potassium, magnesium, sodium salts in combination with proteins are part of the cytoplasm of cells; they determine the acid-base state of the cytoplasm and blood plasma. The excitability of nervous and muscle tissue, enzyme activity, and a number of other important processes occurring in the cell depend on the concentration of certain ions of various salts. Therefore, a cell normally maintains a strictly defined qualitative and quantitative composition of salts.

About 98% of the mass is made up of just four elements. These are oxygen, carbon, hydrogen and nitrogen. The share of oxygen is 65%, carbon – 18%, hydrogen – 10% and nitrogen – 3%. Some scientists are confident that the emergence and existence of terrestrial life apparently became possible only thanks to the unique ability of carbon to form large molecules. In relatively large quantities (tenths and hundredths of a percent) calcium, potassium, silicon, phosphorus, magnesium, sulfur, chlorine, sodium, aluminum, and iron are found in the cell. they, together with the first four (O, C, H and N) form the group of macroelements


Elements grouped into the group of microelements are found in slightly smaller amounts in cells. These are zinc, cobalt, iodine, copper, fluorine, boron, nickel, silver, lithium, chromium and some others. Their content in the cell ranges from thousandths to hundred thousandths of a percent, and the total mass of all microelements is 0.02%.



The supply of water into the cell and the buffering properties of cells and tissues largely depend on salts. Cell membranes are permeable to water molecules and impermeable to large molecules and ions. If the water content in the medium is higher than in the cell, then the equalization of water concentration between the cell and the medium occurs through the penetration of water from the medium into the cell. For example, the absorption of water by plant roots is based on this property. Thus, in the cell, as well as in the body as a whole, there is a clear relationship between various inorganic compounds.

Water is the simplest chemical compound found in living organisms. In terms of quantitative content in the cell, it ranks first - on average it accounts for approximately 75–80%. Water content can vary greatly between cells. Water is found in cells in two states - bound and free. bound free

4–5% of water is in a state bound to protein molecules. This is the so-called solvate water, which forms shells around protein molecules, isolating them from each other and preventing their aggregation. Solvate water differs in its chemical and physical properties from free water. For example, it does not dissolve salts, but freezes at a temperature close to –40°C.

Acts as a solvent for chemicals; is the environment in which vital chemical reactions take place; included as an active component in some enzymatic reactions; carries out the influx of substances into the cell and the removal of waste products from it; determines cell turgor pressure; ensures slight temperature fluctuations inside the cell and uniform distribution of heat throughout the cell and throughout the body. interstitial fluids, consisting mainly of water, wet the integument where friction of one organ occurs on the surface of another. The important role of water is evidenced by a clear connection between the intensity of metabolism and the water content in organs and tissues. 95% of water is in a free state. This water performs the following functions:

Two properties of water - the ability to form hydrogen bonds and reversible ionization - turn out to be very significant for the occurrence of intracellular processes. Oxygen and hydrogen atoms have different electron affinities (electronegativity), and although the water molecule as a whole is electrically neutral, partial negative charges are localized on oxygen, and partially positive charges are localized on hydrogen atoms. Due to this spatial separation of charges, neighboring molecules can be electrostatically attracted to each other. This type of attraction between the partial charges of electrically neutral molecules is called a hydrogen bond.

Organic substances account for 20 to 30% of the cell mass. Organic substances are mainly represented by biopolymers, the molecules of which are large in size and consist of repeatedly repeating elementary units - monomers. The most important biological role belongs to such substances as proteins, nucleic acids, carbohydrates, lipids, hormones, ATP, vitamins, etc. Almost all processes in living organisms are associated with the functioning of proteins and nucleic acids. These are the largest and most complex molecules in the cell, being irregular polymers, i.e. molecules whose functions are significantly determined by the number, composition and order of arrangement of their constituent monomers.

Proteins account for at least half of the dry mass of an animal cell. In living organisms, they perform a wide variety of functions (construction, catalytic, storage, transport, motor, energy, regulatory, protective) and serve as the molecular tools with the help of which genetic information is realized.





In 1868–1870 Swiss biochemist Friedrich Miescher, studying the nuclei of pus cells, discovered a new group of chemical compounds, which he called “nucleins.” These innovations were acidic and contained large amounts of carbon, hydrogen, oxygen, nitrogen and phosphorus. These were nucleic acids - the largest biopolymers. Despite their relatively low content compared to proteins, nucleic acids play a central role in the cell, since their functions are related to the storage and transmission of genetic information. Nucleic acids are linear, irregular polymers. There are two types of nucleic acids, differing in chemical structure and biological properties. These are DNA - deoxyribonucleic acids and RNA - ribonucleic acids. 1) a phosphoric acid residue, 2) a five-carbon monosaccharide in cyclic form - ribose or deoxyribose, 3) a nitrogenous base.


Carbohydrates (saccharides) are the general name for a broad class of natural organic compounds. The name comes from the words “coal” and “water”. The reason for this is that the first carbohydrates known to science were described by the gross formula Cx(H2O)y, formally being compounds of carbon and water.

Simple Monosaccharides - depending on the number of carbon atoms in the monosaccharide molecule, they are distinguished: trioses (3 s), tetroses (4 s), pentoses (5 s), hexoses (6 s), heptoses (7 s). In nature, pentoses and hexoses are the most widespread. The most important of the pentoses are deoxyribose and ribose, which are part of DNA, RNA, ATP; the most common hexoses are glucose, fructose and galactose (general formula CHO). Monosaccharides can be presented as a- and b-isomers. Starch molecules consist of α-glucose residues, while cellulose molecules consist of β-glucose residues. Deoxyribose (CHO) differs from ribose (C H O) in that it has a hydrogen atom at the second carbon atom, rather than a hydroxyl group like ribose.

Complex carbohydrates are those whose molecules, upon hydrolysis, break down to form simple carbohydrates. Among the complex ones there are: oligosaccharides and polysaccharides. Oligosaccharides are complex carbohydrates containing from 2 to 10 monosaccharide residues. Depending on the number of incoming monosaccharide residues included in oligosaccharide molecules, disaccharides, trisaccharides, etc. are distinguished. The most widespread in nature are disaccharides, the molecules of which are formed by two monosaccharide residues: maltose, consisting of two α-glucose residues, milk sugar (lactose) and beet (or sugar) sugar. Polysaccharides are formed as a result of a polycondensation reaction. The most important polysaccharides are starch, glycogen, chitin, murein. Starch is the main reserve carbohydrate in plants, glycogen in animals and humans. Cellulose is the main structural carbohydrate of plant cell walls; it is insoluble in water.

Molecules of simple carbohydrates - monoz - are built from unbranched carbon chains containing different numbers of carbon atoms. The composition of plants and animals includes mainly monoses with 5 and 6 carbon atoms - pentoses and hexoses. The carbon atoms have hydroxyl groups, and one of them is oxidized to an aldehyde (aldose) or ketone (ketose) group. In aqueous solutions, including in a cell, monoses transform from acyclic (aldehyde-ketone) forms into cyclic (furanose, pyranose) forms and back. This process is called dynamic isomerism - tautomerism. The cycles that are part of the monoses molecules can be built from 5 atoms (of which 4 carbon atoms and one oxygen) - they are called furanose, or from 6 atoms (5 carbon atoms and one oxygen), they are called pyranose.

Carbohydrates perform a structural function. Carbohydrates perform a protective role in plants. Carbohydrates perform a plastic function. Carbohydrates are the main energy material. Carbohydrates are involved in providing osmotic pressure and osmoregulation. Carbohydrates perform a receptor function.

The main sources of carbohydrates from food are: bread, potatoes, pasta, cereals, and sweets. Sugar is a pure carbohydrate. Honey, depending on its origin, contains 70-80% sugar. A special bread unit is used to indicate the amount of carbohydrates in food. In addition, the carbohydrate group also includes fiber and pectins, which are poorly digestible by the human body.

Carbohydrates are necessary in the daily diet so that the protein needed for tissue building is not wasted as a source of energy where it is needed for recovery. They have the same calorie content as protein. If you eat too many carbohydrates, more than can be converted into glucose or glycogen (which is stored in the liver and muscles), the result, as we all know too well, is fat. When the body needs more fuel, fat is converted back to glucose and body weight decreases. 36


Lipids are natural compounds that are obtained from plant or animal tissues by extraction with non-polar solvents (for example, ether, benzene or chloroform) and that are insoluble in water. These include the products of interaction of fatty acids with alcohols (simple lipids), amino alcohols and other compounds (complex lipids), prostaglandins and isoprenoid lipids (for example, carotenoids, chlorophyll, vitamins E and K). Depending on the cell type, the lipid content ranges from 5 to 90% (in adipose tissue cells). These are hydrophobic substances with high energy intensity (the breakdown of 1 g of fat gives 38.9 kJ).

Presentation on the topic: Presentation of the chemical composition of cells.
1 slide:
Chemical composition of the cell and its structure
2 slide
Contents 1. Chemical composition of the cell: * Inorganic compounds (water and mineral salts) * Carbohydrates * Lipids (fats) * Proteins * Nucleic acids: DNA and RNA * ATP and other organic compounds (hormones and vitamins) 2. Structure and functions of the cell: * Cell theory * Cytoplasm and Biological membrane * Endoplasmic reticulum and Ribosomes * Golgi complex and Lysosomes * Mitochondria, Organelles of movement and inclusions * Plastids * Nucleus. Prokaryotes and eukaryotes
3 slide
General information The chemical composition of plant and animal cells is very similar, which indicates the unity of their origin. More than 80 chemical elements have been found in cells, but only 27 of them have a known physiological role. Macroelements: O, C, N, H. 98% Microelements: K, P, S, Ca, Mg, Cl, Na. 1.9% Ultramicroelements: Cu, I, Zn, Co, Br. 0.01%
4 slide
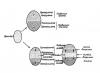
Inorganic compounds The most common inorganic compound in the cells of living organisms is water. It enters the body from the external environment; in animals, in addition, it can be formed during the breakdown of fats, proteins, and carbohydrates. Water is found in the cytoplasm and its organelles, vacuoles, nucleus, and intercellular spaces. Functions: 1. Solvent 2. Transport of substances 3. Creation of an environment for chemical reactions 4. Participation in the formation of cellular structures (cytoplasm)
5 slide
Inorganic compounds Mineral salts in certain concentrations are necessary for the normal functioning of cells. For example, insoluble calcium and phosphorus salts ensure the strength of bone tissue. The content of cations and anions in the cell and its surrounding environment (blood plasma, intercellular substance) is different due to the semi-permeability of the membrane.
6 slide
Carbohydrates are organic compounds containing hydrogen (H), carbon (C) and oxygen (O). Carbohydrates are formed from water (H2O) and carbon dioxide (CO2) during photosynthesis. Fructose and glucose are constantly present in the cells of plant fruits, giving them a sweet taste. Functions: 1. Energy (during the breakdown of 1 g of glucose, 17.6 kJ of energy is released) 2. Structural (chitin in the skeleton of insects and in the cell wall of fungi) 3. Storage (starch in plant cells, glycogen in animals)
7 slide
Lipids A group of fat-like organic compounds, insoluble in water, but highly soluble in non-polar organic solvents (benzene, gasoline, etc.). Lipoproteins, glycolipids, phospholipids. Fats are one of the classes of lipids, esters of glycerol and fatty acids. The cells contain from 1 to 5% fat. Functions: 1. Energy (the oxidation of 1 g of fat releases 38.9 kJ of energy) 2. Structural (phospholipids are the main elements of cell membranes) 3. Protective (thermal insulation)
8 slide
Proteins These are biopolymers whose monomers are amino acids. In the structure of a protein molecule, a primary structure is distinguished - the sequence of amino acid residues; The secondary is a helical structure that is held together by many hydrogen bonds. The tertiary structure of a protein molecule is a spatial configuration resembling a compact globule. It is supported by ionic, hydrogen and disulfide bonds, as well as hydrophobic interactions. The quaternary structure is formed by the interaction of several globules (for example, the hemoglobin molecule consists of four such subunits). The loss of a protein molecule's natural structure is called denaturation.
Slide 9
Nucleic acids Nucleic acids provide storage and transmission of hereditary (genetic) information in living organisms. DNA (deoxyribonucleic acid) is a molecule consisting of two helically twisted polynucleotide chains. The DNA monomer is a deoxyribonucleotide, consisting of a nitrogenous base (adenine (A), cytosine (C), thymine (T) or guanine (G)), pentose (deoxyribose) and phosphate. RNA (ribonucleic acid) is a molecule consisting of a single chain of nucleotides. A ribonucleotide consists of one of four nitrogenous bases, but instead of thymine (T) in RNA there is uracil (U), and instead of deoxyribose there is ribose.
10 slide
ATP ATP (adenosine triphosphoric acid) is a nucleotide belonging to the group of nucleic acids. The ATP molecule consists of the nitrogenous base adenine, the five-carbon monosaccharide ribose and three phosphoric acid residues, which are connected to each other by high-energy bonds. The cleavage of one molecule of phosphoric acid occurs with the help of enzymes and is accompanied by the release of 40 kJ of energy. The cell uses ATP energy in biosynthesis processes, during movement, during heat production, during nerve impulses, during photosynthesis, etc. ATP is a universal energy accumulator in living organisms
11 slide
Cell theory In 1665, the English naturalist Robert Hooke, observing a section of wood cork under a microscope, discovered empty cells, which he called “cells.” Modern cell theory includes the following provisions: *all living organisms consist of cells; cell - the smallest unit of living things; * the cells of all unicellular and multicellular organisms are similar in their structure, chemical composition, basic manifestations of life activity and metabolism; * cell reproduction occurs by dividing them, and each new cell is formed as a result of the division of the original (mother) cell; all multicellular organisms develop from one cell * in complex multicellular organisms, cells are specialized in the function they perform and form tissues; tissues consist of organs that are closely interconnected and subordinate to nervous and humoral regulatory systems.
The cells of living organisms differ from each other not only in structure and functions, but also in chemical composition. Different cells contain almost the same chemical elements.
There are about 80 in a cell chemical elements Periodic table of Dmitry Ivanovich Mendeleev. These are almost all the elements that are present on our planet and are known today. The function of these elements has been little studied, since out of 80 elements only 24 have a defined function that they perform in the cell.
Chemical elements that are found in the cell are divided into three large groups: macronutrients , microelements And ultramicroelements.
The distribution of chemical elements in the cell is uneven. The majority, approximately 98%, of the mass of any cell is made up of macronutrients. First of all, it is oxygen (75%), carbon (15%), hydrogen (8%), nitrogen (3%). The molecules of organic substances are made up of these elements, and oxygen and hydrogen are part of water, which is the main inorganic substance of the cell. Macroelements also include phosphorus, potassium, sulfur, iron, magnesium, sodium and calcium. The mass fraction of any macroelement in the cell is at least 0.001%.
Chemical elements that account for from 0.001% to 0.000001% (read: from 1 thousandth to 1 millionth of a percent) in a cell are called microelements. These are zinc, iodine, copper, manganese, fluorine, cobalt, bromine and others.
The percentage content of a particular element in the body in no way characterizes the degree of its importance and necessity in the body.
For example, cobalt is part of vitamin B12, iodine is part of the hormones thyroxine and thyronine, and copper is part of enzymes that catalyze redox processes. In addition, copper is involved in the transport of oxygen in the tissues of mollusks. A significant number of enzymes with a diverse mechanism of action contain ions of zinc, manganese, cobalt and molybdenum.
Silicon is found in diatoms, horsetails, sponges and mollusks. In the cartilages and ligaments of vertebrates, its content can reach several hundredths of a percent.
Boron affects plant growth, fluorine is part of the enamel of teeth and bones.
Per share ultramicroelements accounts for less than 0.000001% of the cell mass. This group includes radium, cesium, mercury, uranium, gold and others.
All cell substances are divided into two groups: inorganic And organic.
The main inorganic substance of the cell is water. Due to its physicochemical properties, water is a good solvent, therefore, it is a medium for chemical reactions to occur in the cell. Due to the polarity of the molecules, water easily dissolves ionic compounds (salts, acids, bases). Substances that are highly soluble in water are called hydrophilic. Fats, nucleic acids and some proteins are poorly soluble in water or not at all. Such substances are called hydrophobic.
Water plays an important role in the life of organisms due to its properties:
Thanks to the high heat capacity, water is capable of absorbing thermal energy with a minimal increase in its own temperature. The release of water (transpiration in plants, sweating in animals) protects the body from overheating.
Possessing high thermal conductivity, water promotes uniform distribution of heat throughout the body.
Practically without shrinking, water creates turgor pressure, which determines the volume and elasticity of cells.
Due to the formation of hydrogen bonds between water molecules and molecules of other substances, water has an optimal value for biological systems surface tension forces, thanks to which capillary blood flow and movement of solutions in plants is carried out.
Mineral salts in a cell they can be in dissolved or undissolved states. Soluble salts dissociate into ions. The most important cations are:
potassium And sodium, which are responsible for the transport of substances across the cell membrane and are involved in the occurrence and conduction of nerve impulses;
calcium takes part in the processes of muscle fiber contraction and blood clotting. Insoluble calcium salts are involved in the formation of bones and teeth, calcium carbonate - in the formation of mollusk shells, strengthening the cell membranes of some plant species;
magnesium is part of chlorophyll;
iron is part of a number of proteins, including hemoglobin.
Zinc is part of the molecule of the pancreatic hormone - insulin, copper participates in the processes of photosynthesis and respiration.
The most important anions are phosphate anion, which is part of ATP and nucleic acids, and carbonic acid residue regulating fluctuations in the pH of the environment.
Organic matter cells are represented by carbohydrates, lipids, proteins, nucleic acids, ATP, vitamins and hormones.
1 slide
Topic: “Chemical composition of the cell. Inorganic substances of the cell" Objectives: To characterize the chemical composition of the cell: groups of elements that make up the cell; Reveal the properties and importance of water, the role of the most important cations and anions in the cell. Chapter I. Chemical composition of the cell Pimenov A.V.

2 slide
All living organisms on Earth are divided into two empires - the Cellular Empire and the Non-Cellular Empire. The Cellular Empire unites organisms that have a cellular structure. Non-cellular organisms include viruses, grouped under the kingdom Viruses. Properties of living organisms

3 slide
1. The most important feature of a living organism is the ability to reproduce, the ability to transmit genetic information to the next generation. With asexual reproduction, the next generation receives genetic information from the mother's body; with sexual reproduction, the genetic information of two organisms is combined. 2. A living organism is an open system; it receives nutrients, it uses various types of energy - light energy, energy released during the oxidation of organic and inorganic substances, and releases metabolic products and energy into the environment. In other words, there is a constant exchange of substances and energy between the body and its environment. 3. The cells of living organisms are formed by various biopolymers, the most important of which are nucleic acids and proteins. But a dead horse is also composed of biopolymers, so it is important to emphasize their constant self-renewal. 4. While the body is alive, it perceives environmental influences, under the influence of the stimulus, excitement occurs and a response to the excitement develops. Excitability is the most important property of the body. Properties of living organisms

4 slide
5. As a result of natural selection, organisms have amazingly adapted to specific living conditions. This adaptation began with evolution at the molecular level, then at the level of cell organelles - at the cellular level, then at the level of a multicellular organism. 6. Living organisms are characterized by a high degree of organization, which is manifested in the complex structure of biological molecules, organelles, cells, organs, and their specialization to perform certain functions. 7. Also, the signs of living organisms include growth, aging and death. Properties of living organisms

5 slide
Scientists, based on the characteristics of the manifestation of the properties of living things, distinguish several levels of organization of living nature: Molecular. Cellular. Organic. Population-species. Ecosystem. Biosphere. Levels of organization of living matter

6 slide
The molecular level is represented by molecules of organic substances - proteins, carbohydrates, lipids, nucleic acids, located in cells and called biological molecules. Levels of organization of living matter

7 slide
At the cellular level, the structure of cells, the structure and functions of its individual organelles are studied. Levels of organization of living matter

8 slide
At the organismal level - the structure of tissues, organs and organ systems of the entire organism. Levels of organization of living matter

Slide 9
At the population-species level, the structure of the species and the characteristics of populations are studied. Levels of organization of living matter

10 slide
At the ecosystem (biogeocoenotic) level, the structure and characteristics of biogeocenoses are studied. Levels of organization of living matter

11 slide

12 slide
What is being studied at the molecular level? The molecules of organic substances are studied - proteins, carbohydrates, lipids, nucleic acids found in cells and called biological molecules. What is being studied at the cellular level? At the cellular level, the structure of cells, the structure and functions of its individual organelles are studied. What is being studied at the organismal level? The structure of tissues, organs and organ systems of the whole organism. What is being studied at the population-species level? At the population-species level, the structure of the species and the characteristics of populations are studied. What is studied at the biogeocenotic level? At the ecosystem (biogeocoenotic) level, the structure and characteristics of biogeocenoses are studied. What is being studied at the biosphere level? At the biosphere level, the biosphere is studied. Distribution of life in the atmosphere, lithosphere, hydrosphere. Human influence on the biosphere. Let's summarize:

Slide 13

Slide 14
Chemical composition of the cell All cells, regardless of the level of organization, are similar in chemical composition. About 80 chemical elements of D.I. Mendeleev’s periodic table were discovered in living organisms. For 24 elements, the functions they perform in the cell are known. These elements are called biogenic. According to their quantitative content in living matter, elements are divided into three categories: Macroelements: O, C, H, N - about 98% of the cell mass, elements of the 1st group; K, Na, Ca, Mg, S, P, Cl, Fe - 1.9% of the cell mass, elements of the 2nd group. Macroelements include elements whose concentration exceeds 0.001%. They make up the bulk of the living matter of the cell. Microelements: (Zn, Mn, Cu, Co, Mo and many others), the proportion of which ranges from 0.001% to 0.000001% (0.1% of cell mass). They are part of biologically active substances - enzymes, vitamins and hormones. Ultramicroelements: (Au, U, Ra, etc.), the concentration of which does not exceed 0.000001%. The role of most elements of this group has not yet been clarified.

15 slide

16 slide

Slide 17
What elements belong to the elements of group 1? S, N, O, N.. Which elements belong to the elements of the 2nd group? : K, Na, Ca, Mg, S, P, Cl, Fe. What percentage of the mass are elements of groups 1 and 2: Elements of group 1 - 98%, elements of group 2 - 2%. What elements are called macronutrients? Elements whose amount is more than 0.001% of body weight are called macroelements. What elements are called micro- and ultramicroelements? Elements whose share is from 0.001 to 0.000001% are microelements, and elements whose content does not exceed 0.000001% are ultramicroelements. Let's summarize:

18 slide

Slide 19
Water. The most common inorganic compound in living organisms. Its content varies widely: in the cells of tooth enamel, water makes up about 10% by weight, and in the cells of a developing embryo - more than 90%. Chemical compounds of the cell. Water

20 slide
A water molecule consists of an O atom linked to two H atoms by polar covalent bonds. The characteristic arrangement of electrons in a water molecule gives it electrical asymmetry. The more electronegative oxygen atom attracts the electrons of the hydrogen atoms more strongly, as a result of which the common pairs of electrons in the water molecule are shifted towards it. Therefore, although the water molecule as a whole is uncharged, each of the two hydrogen atoms carries a partially positive charge (denoted δ+), and the oxygen atom carries a partially negative charge (2δ-). The water molecule is polarized and is a dipole (has two poles). Chemical compounds of the cell. Water

21 slides
The partially negative charge of the oxygen atom of one water molecule is attracted by the partially positive hydrogen atoms of other molecules. Thus, each water molecule tends to hydrogen bond with four neighboring water molecules. Water is a good solvent. Due to the polarity of molecules and the ability to form hydrogen bonds, water easily dissolves ionic compounds (salts, acids, bases). Some non-ionic but polar compounds are also soluble in water, i.e., the molecule of which contains charged (polar) groups, for example sugars, simple alcohols, amino acids. Substances that are highly soluble in water are called hydrophilic (from the Greek hygros - wet and philia - friendship, inclination). Chemical compounds of the cell. Water

22 slide

Slide 23
Substances that are poorly or completely insoluble in water are called hydrophobic (from the Greek phobos - fear). These include fats, nucleic acids, and some proteins. Such substances can form interfaces with water at which many chemical reactions take place. Therefore, the fact that water does not dissolve non-polar substances is also very important for living organisms. Among the physiologically important properties of water is its ability to dissolve gases (O2, CO2, etc.). Chemical compounds of the cell. Water

24 slide
Water has a high heat capacity, i.e. the ability to absorb thermal energy with a minimal increase in its own temperature. The large heat capacity of water protects body tissues from rapid and strong temperature increases. Many organisms cool themselves by evaporating water (transpiration in plants, sweating in animals). Chemical compounds of the cell. Water
28 slide
The most important anions: H2PO4-, HPO42-, HCO3-, Cl- Buffering – the ability to maintain pH at a certain level. A pH value of 7.0 corresponds to a neutral solution, below 7.0 to an acidic solution, and above 7.0 to an alkaline solution. In the cell pH = 7.4. Chemical compounds of the cell. Salts

Slide 29
What substances are considered hydrophilic substances? Water easily dissolves ionic compounds (salts, acids, bases). Some non-ionic but polar compounds are also soluble in water, i.e., the molecule of which contains charged (polar) groups, for example sugars, simple alcohols, amino acids. Why are lipids insoluble in water? Lipid molecules have no charge and do not hydrate. Why is water classified as a substance with high heat capacity? What does this mean for organisms? Water is capable of absorbing thermal energy with a minimal increase in its own temperature. The large heat capacity of water protects body tissues from rapid and strong temperature increases. How is heat transfer regulated using water? Many organisms cool themselves by evaporating water (transpiration in plants, sweating in animals). What is the significance of high thermal conductivity of water? Provides uniform distribution of heat throughout the body. Why is solid ice lighter than liquid water? The density of water in the solid state is less than in the liquid state, due to which ice forms on the surface of the water. Let's summarize:

Slide 1
 Slide 2
Slide 2
 Slide 3
Slide 3
 Slide 4
Slide 4
 Slide 5
Slide 5
 Slide 6
Slide 6
 Slide 7
Slide 7
 Slide 8
Slide 8
 Slide 9
Slide 9
 Slide 10
Slide 10
 Slide 11
Slide 11
 Slide 12
Slide 12
 Slide 13
Slide 13
 Slide 14
Slide 14
 Slide 15
Slide 15
 Slide 16
Slide 16
 Slide 17
Slide 17
 Slide 18
Slide 18
 Slide 19
Slide 19
 Slide 20
Slide 20
 Slide 21
Slide 21
 Slide 22
Slide 22
The presentation on the topic “The chemical composition of the cell and its structure” can be downloaded absolutely free of charge on our website. Project subject: Biology. Colorful slides and illustrations will help you engage your classmates or audience. To view the content, use the player, or if you want to download the report, click on the corresponding text under the player. The presentation contains 22 slide(s).
Presentation slides

Slide 1

Slide 2
1. Chemical composition of the cell: * Inorganic compounds (water and mineral salts) * Carbohydrates * Lipids (fats) * Proteins * Nucleic acids: DNA and RNA * ATP and other organic compounds (hormones and vitamins) 2. Structure and functions of the cell: * Cell theory * Cytoplasm and Biological membrane * Endoplasmic reticulum and Ribosomes * Golgi complex and Lysosomes * Mitochondria, Organelles of movement and inclusions * Plastids * Nucleus. Prokaryotes and eukaryotes

Slide 3
General information
The chemical composition of plant and animal cells is very similar, which indicates the unity of their origin. More than 80 chemical elements have been found in cells, but only 27 of them have a known physiological role. Macroelements: O, C, N, H. 98% Microelements: K, P, S, Ca, Mg, Cl, Na. 1.9% Ultramicroelements: Cu, I, Zn, Co, Br. 0.01%

Slide 4
Inorganic compounds
The most common inorganic compound in the cells of living organisms is water. It enters the body from the external environment; in animals, in addition, it can be formed during the breakdown of fats, proteins, and carbohydrates. Water is found in the cytoplasm and its organelles, vacuoles, nucleus, and intercellular spaces. Functions: 1. Solvent 2. Transport of substances 3. Creation of an environment for chemical reactions 4. Participation in the formation of cellular structures (cytoplasm)

Slide 5
Mineral salts in certain concentrations are necessary for the normal functioning of cells. For example, insoluble calcium and phosphorus salts ensure the strength of bone tissue. The content of cations and anions in the cell and its surrounding environment (blood plasma, intercellular substance) is different due to the semi-permeability of the membrane.

Slide 6
Carbohydrates
These are organic compounds that contain hydrogen (H), carbon (C) and oxygen (O). Carbohydrates are formed from water (H2O) and carbon dioxide (CO2) during photosynthesis. Fructose and glucose are constantly present in the cells of plant fruits, giving them a sweet taste. Functions: 1. Energy (during the breakdown of 1 g of glucose, 17.6 kJ of energy is released) 2. Structural (chitin in the skeleton of insects and in the cell wall of fungi) 3. Storage (starch in plant cells, glycogen in animals)

Slide 7
A group of fat-like organic compounds, insoluble in water, but highly soluble in non-polar organic solvents (benzene, gasoline, etc.). Lipoproteins, glycolipids, phospholipids. Fats are one of the classes of lipids, esters of glycerol and fatty acids. The cells contain from 1 to 5% fat. Functions: 1. Energy (the oxidation of 1 g of fat releases 38.9 kJ of energy) 2. Structural (phospholipids are the main elements of cell membranes) 3. Protective (thermal insulation)

Slide 8
These are biopolymers whose monomers are amino acids. In the structure of a protein molecule, a primary structure is distinguished - the sequence of amino acid residues; The secondary is a helical structure that is held together by many hydrogen bonds. The tertiary structure of a protein molecule is a spatial configuration resembling a compact globule. It is supported by ionic, hydrogen and disulfide bonds, as well as hydrophobic interactions. The quaternary structure is formed by the interaction of several globules (for example, the hemoglobin molecule consists of four such subunits). The loss of a protein molecule's natural structure is called denaturation.

Slide 9
Nucleic acids
Nucleic acids provide storage and transmission of hereditary (genetic) information in living organisms. DNA (deoxyribonucleic acid) is a molecule consisting of two helically twisted polynucleotide chains. The DNA monomer is a deoxyribonucleotide, consisting of a nitrogenous base (adenine (A), cytosine (C), thymine (T) or guanine (G)), pentose (deoxyribose) and phosphate. RNA (ribonucleic acid) is a molecule consisting of a single chain of nucleotides. A ribonucleotide consists of one of four nitrogenous bases, but instead of thymine (T) in RNA there is uracil (U), and instead of deoxyribose there is ribose.

Slide 10
ATP (adenosine triphosphoric acid) is a nucleotide belonging to the group of nucleic acids. The ATP molecule consists of the nitrogenous base adenine, the five-carbon monosaccharide ribose and three phosphoric acid residues, which are connected to each other by high-energy bonds. The cleavage of one molecule of phosphoric acid occurs with the help of enzymes and is accompanied by the release of 40 kJ of energy. The cell uses ATP energy in biosynthesis processes, during movement, during heat production, during nerve impulses, during photosynthesis, etc. ATP is a universal energy accumulator in living organisms

Slide 11
Cell theory
In 1665, the English naturalist Robert Hooke, observing a section of wood cork under a microscope, discovered empty cells, which he called “cells.” Modern cell theory includes the following provisions: *all living organisms consist of cells; cell - the smallest unit of living things; * the cells of all unicellular and multicellular organisms are similar in their structure, chemical composition, basic manifestations of life activity and metabolism; * cell reproduction occurs by dividing them, and each new cell is formed as a result of the division of the original (mother) cell; all multicellular organisms develop from one cell * in complex multicellular organisms, cells are specialized in the function they perform and form tissues; tissues consist of organs that are closely interconnected and subordinate to nervous and humoral regulatory systems.

Slide 12
Cytoplasm Biological membrane
A semi-liquid medium in which the cell nucleus and all organelles are located. The cytoplasm is 85% water and 10% protein. The biological membrane delimits the contents of the cell from the external environment, forms the walls of most organelles and the shell of the nucleus, and divides the contents of the cytoplasm into separate compartments. The outer and inner layers of the membrane (dark) are formed by protein molecules, and the middle (light) by two layers of lipid molecules. Lipid molecules are arranged in a strictly ordered manner: the water-soluble (hydrophilic) ends of the molecules face the protein layers, and the water-insoluble (hydrophobic) ends face each other. The biological membrane has selective permeability.

Slide 13
Endoplasmic reticulum (ER)
This is a network of channels, tubes, vesicles, cisterns located inside the cytoplasm. EPS is a system of membranes with an ultramicroscopic structure. There are smooth (agranular) and rough (granular) ER, which carries ribosomes. On the membranes of the smooth ER there are enzyme systems involved in fat and carbohydrate metabolism. Ribosomes are attached to the membrane of the granular ER, and during the synthesis of a protein molecule, the polypeptide chain from the ribosome is immersed in the ER channel

Slide 14
Ribosomes
Small spherical organelles ranging in size from 15 to 35 nm, consisting of two unequal subunits and containing approximately equal amounts of protein and RNA. Most of the ribosomal subunits are synthesized in the nucleoli and enter the cytoplasm through the pores of the nuclear membrane, where they are located either on the membranes of the endoplasmic reticulum or freely. During protein synthesis, they can be combined on messenger RNA into groups (polysomes)

Slide 15
Golgi complex
The Golgi complex is a stack of 5-10 flat cisterns, along the edges of which branching tubes and small vesicles extend. It is part of the membrane system: outer membrane of the nuclear envelope - endoplasmic reticulum - Golgi complex - outer cell membrane. In this system, the synthesis and transfer of various compounds occurs, as well as substances secreted by the cell in the form of secretions or waste. The Golgi complex takes part in the formation of lysosomes, vacuoles, the accumulation of carbohydrates, and the construction of the cell wall (in plants).

Slide 16
Lysosomes
Spherical bodies covered with an elementary membrane and containing about 30 hydrolytic enzymes capable of breaking down proteins, nucleic acids, fats and carbohydrates. The formation of lysosomes occurs in the Golgi complex. If the membranes of lysosomes are damaged, the enzymes they contain can destroy the structures of the cell itself and temporary organs of embryos and larvae, for example, the tail and gills during the development of frog tadpoles.

Slide 17
Plastids
Contained only in plant cells. Chloroplasts are shaped like a biconvex lens and contain the green pigment chlorophyll. Chloroplasts have the ability to capture sunlight and use it to synthesize organic substances with the participation of ATP. Chromoplasts are plastids containing plant pigments (except green) that give color to flowers, fruits, stems and other parts of plants. Leukoplasts are colorless plastids, most often found in uncolored parts of plants - roots, bulbs, etc. They can synthesize and accumulate proteins, fats and polysaccharides (starch).

Slide 18
Mitochondria
Visible under a light microscope in the form of granules, rods, threads ranging in size from 0.5 to 7 microns. The wall of mitochondria consists of two membranes - the outer, smooth one and the inner one, which forms projections - cristae, which protrude into the internal contents of the mitochondria (matrix). The matrix contains an autonomous protein biosynthesis system: mitochondrial RNA, DNA and ribosomes. The main functions of mitochondria are the oxidation of organic compounds to carbon dioxide and water and the accumulation of chemical energy in high-energy bonds of ATP.

Slide 19
Organelles of movement Inclusions
Cellular organelles of movement include cilia and flagella - these are membrane outgrowths with a diameter containing microtubules in the middle. The function of these organelles is either to provide movement (for example, in protozoa) or to move fluid along the surface of cells (for example, in the respiratory epithelium to move mucus). Inclusions are unstable components of the cytoplasm, the content of which varies depending on the functional state of the cell. .

Slide 20
The shape and size of the nucleus depend on the shape and size of the cell and the function it performs. In terms of chemical composition, the nucleus differs from other components of the cell in its high content of DNA (15-30%) and RNA (12%). 99% of the cell's DNA is concentrated in the nucleus, where it, together with proteins, forms complexes - deoxyribonucleoproteins (DNP). The nucleus performs two main functions: 1) storage and reproduction of hereditary information; 2) regulation of metabolic processes occurring in the cell. The nucleus consists of a nucleolus, consisting of protein and r-RNA; chromatin (chromosomes) and nuclear juice, which is a colloidal solution of proteins, nucleic acids, carbohydrates and enzymes, mineral salts.

Tips for making a good presentation or project report
- Try to involve the audience in the story, set up interaction with the audience using leading questions, a game part, do not be afraid to joke and smile sincerely (where appropriate).
- Try to explain the slide in your own words, add additional interesting facts; you don’t just need to read the information from the slides, the audience can read it themselves.
- There is no need to overload the slides of your project with text blocks; more illustrations and a minimum of text will better convey information and attract attention. The slide should contain only key information; the rest is best told to the audience orally.
- The text must be well readable, otherwise the audience will not be able to see the information being presented, will be greatly distracted from the story, trying to at least make out something, or will completely lose all interest. To do this, you need to choose the right font, taking into account where and how the presentation will be broadcast, and also choose the right combination of background and text.
- It is important to rehearse your report, think about how you will greet the audience, what you will say first, and how you will end the presentation. All comes with experience.
- Choose the right outfit, because... The speaker's clothing also plays a big role in the perception of his speech.
- Try to speak confidently, smoothly and coherently.
- Try to enjoy the performance, then you will be more at ease and less nervous.